Fig. 22.1
A 50-year-old female presenting with biopsy-proven recurrent epitheliod hemangioendothelioma 6 years post living-related liver transplantation measuring 3.8 × 2.9 cm (a) underwent an uneventful nonanatomic wedge resection of the lesion with adequate margins. The patient presented to the hospital on postoperative day 12 with worsening right upper quadrant pain, subjective fevers, and chills. A CT examination demonstrated a 7.8 × 4.6 cm hypoattenuating fluid collection in the resection bed with a few small foci of scattered gas without significant rim enhancement (b). The patient’s white blood cell count was not elevated at 8.8 K/uL; however, due to her immunosuppression and presenting symptoms there was concern for postoperative infection. The patient was initiated on broad-spectrum antibiotics and the fluid collection was subsequently aspirated under US guidance where it demonstrated a complex appearance with predominantly hypoechoic appearing fluid with echogenic debris (c). The collection was completely aspirated with removal of approximately 60 mL of dark bilious appearing fluid (d). The Gram stain and culture were negative and the patient did not require any further management
Prior to minimally invasive image-guided therapies, assessment should include evaluation of coagulation parameters with a target INR < 1.5, aPTT of less than 1.5x control, and platelet count > 50,000/µL with correction of these parameters on a case by case basis [44]. Ultrasound or CT guidance can be used for aspiration or catheter drainage based on operator preference. When choosing a puncture path, the least amount of hepatic parenchyma should be traversed, and care should be taken to avoid damaging adjacent organs or traversing the pleura due to the risk of empyema (See Fig. 22.2). Ultrasound is the preferred imaging modality in most cases due to real-time guidance, multiplanar imaging, portability, visualization, and avoidance of major blood vessels and pleura/lung, and lack of ionizing radiation. Ultrasound-guided procedures can be performed via a subcostal or intercostal approach. A subcostal approach is generally favored over an intercostal puncture due to a lower risk of pneumothorax, empyema, or intercostal artery injury. Sonographically guided interventions can be performed using either a free-hand technique (which provides for greater freedom in needle placement), or with an attached biopsy guide which provides greater accuracy. Local anesthetic should be liberally applied from the skin entry site down through the subcutaneous fat and peritoneum directly adjacent to the fluid collection. If possible, the needle should be placed during a breath hold to reduce the risk of capsule laceration and to facilitate needle entry at the site of local anesthetic administration. Visualization of any adjacent large vascular structures can also be assessed with Doppler US prior to needle placement. CT guidance may be beneficial in scenarios where sonographic visualization is limited by either appearance of the collection on US, adjacent wound complications necessitating a different trajectory, and also to confirm appropriate needle placement utilizing US guidance (See Fig. 22.3). Most interventional CT units are capable of CT fluoroscopy and gantry angle adjustment which aid in procedural planning. Needle aspiration is usually performed with an 18-gauge needle and samples should be appropriately sent for microbiologic analysis. During attempted needle aspiration, if the fluid is too viscous for adequate drainage or is frankly purulent, a catheter can be placed in the same setting utilizing the Seldinger technique. Catheter drainage may also be performed utilizing the trocar technique and a multi-side hole, locking catheter of various sizes can be placed. Abscess drainage catheter monitoring and care is of critical importance to ensure adequate drainage. Abscess catheters are usually flushed up to three times daily with sterile normal saline to prevent clogging. The output from the catheter should be recorded on a daily or per shift basis, and the presence of high outputs is suggestive of a fistula to the cavity. Clinical parameters such as drain output, hemodynamic status, leukocyte count, and culture results should be followed on a daily basis in the early postprocedure period to evaluate the patient’s clinical progress. Catheters can be placed to either suction or gravity drainage. Passive drainage may minimize catheter occlusion secondary to aspirated debris within the abscess cavity; however, active drainage (suction) may result in more rapid evacuation of the abscess with opposition of the abscess cavity wall. Patients who do not respond clinically to percutaneous drainage catheter placement should be further evaluated with cross-sectional imaging, preferably CT, to assess the adequacy of catheter placement and/or the development of new potential sites of infection. In the event of catheter malfunction or inadequate drainage of the collection, the drainage catheter can be exchanged over a wire for larger bore catheters; however, in some cases of significant loculation or debris, more than one catheter may be required for adequate percutaneous management. Another option to aid in the success of percutaneous abscess drainage is the administration of thrombolytic agents through the abscess drainage catheter. If follow-up imaging demonstrates a persistent abscess cavity despite optimal drain placement and sizing, tissue-type plasminogen activator (tPA) can be instilled into the cavity to promote further drainage. Common practice is to dilute 4–6 mg of tPA in 25 mL of sterile 0.9 % normal saline and infuse through the catheter and allow it to dwell for 30 min–1 h. Afterward, the drainage catheter is replaced to passive or active drainage, and the outputs from the catheter are monitored closely. This technique can be performed from once per day up to three times per day as long as it is effective. Thrombolytic therapy in abscess cavities can be effective due to the presence of a fibrin matrix within the cavity which when administered can result in breakdown of loculations and reduction of the viscosity of the fluid within the collection. Thrombolytic therapy has proved to be a safe and effective therapy even in the postoperative period with minimal to no risk of bleeding [45]. Drainage is usually continued until the patient demonstrates clinical improvement and drainage output is less than 10–20 mL/day [46]. The length of time required for successful percutaneous catheter drainage is highly variable and dependent on multiple patient and infection site factors. A fluoroscopic abscessogram can be performed prior to catheter removal to assess the residual size of the cavity and the presence of fistulization to the bowel or biliary system if indicated.
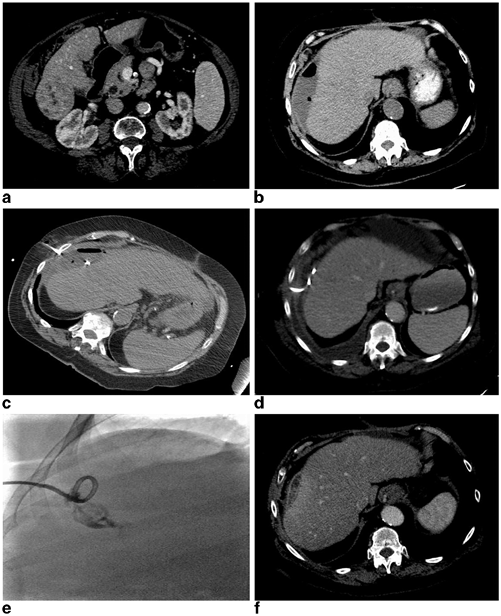
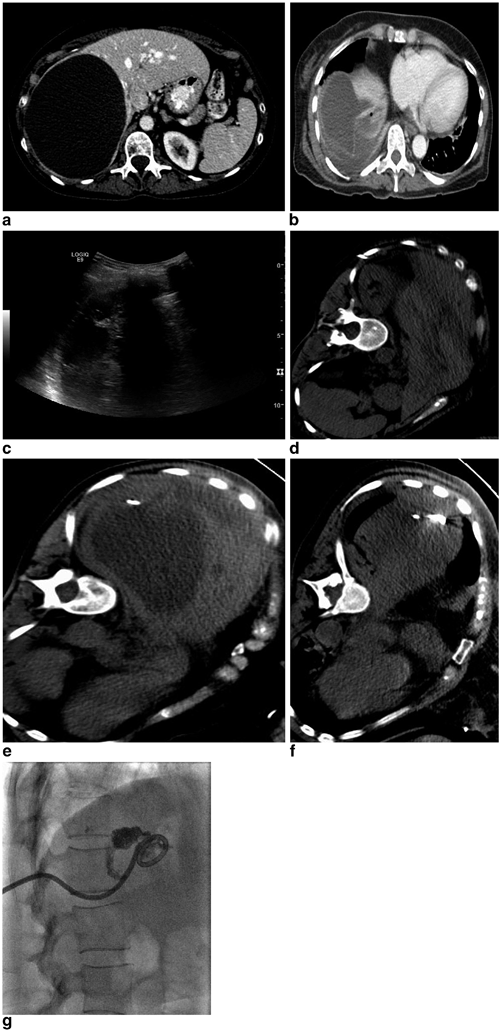

Fig. 22.2
A 68-year-old female with history of cirrhosis secondary to NASH and alcoholism, Child Pugh class A, insulin-dependent diabetes mellitus, and coronary artery disease who presented with a 5.6 cm arterially enhancing exhophytic mass in segment 6 of the liver with portal venous washout consistent with hepatocellular carcinoma (a). The patient underwent an uneventful nonanatomic wedge resection, which revealed a well-differentiated HCC without vascular invasion. Her postoperative course was remarkable for postoperative bleeding which was managed with transfusion of two units of packed red blood cells and she was discharged on postoperative day number 8. She presented with increasing right upper quadrant pain approximately 8 weeks after surgery and laboratory evaluation revealed a normal white blood cell count. A CT of the abdomen and pelvis demonstrated a 10.8 × 3.5 cm rim enhancing hypoattenuating fluid collection with an air-fluid level along the right lateral liver (b). CT guidance was utilized for an intercostal approach into the collection and a 10 Fr locking loop drainage catheter was placed with aspiration of 60 mL of purulent fluid, which grew Enterobacter cloacae and Escherichia coli (c). She was discharged on appropriate antibiotic therapy, however, presented to the hospital 2.5 weeks after percutaneous drainage catheter placement with altered mental status. A CT examination demonstrated near-complete resolution of the abscess cavity; however, the drainage catheter was noted to cross the pleura and there was a new pleural effusion with enhancement (d). A diagnostic thoracentesis was performed with aspiration of 260 mL of serosanguinous fluid, which grew methicillin-resistant staphylococcus aureus (images not shown). The patient’s antibiotic regimen was adjusted accordingly and an abscessogram was performed demonstrating no significant residual collection or evidence of biliary or bowel fistula and the catheter was removed (e). A follow-up CT examination 6 weeks status post drainage catheter removal demonstrates no residual pleural fluid and a small amount of residual inflammatory changes in the abscess cavity with no evidence of residual or recurrent disease (f)

Success rates for image-guided needle aspiration of simple pyogenic liver abscesses less than 5 cm in size approach 100 % with minimal complications [38, 41, 42]. Catheter drainage success rates have varied significantly in the literature from 66 to 100 % likely secondary to abscess and patient factors. Higher failure rates have been associated with the presence of advanced malignancy, particularly necrotic infected tumors, and the presence of fistulization to an obstructed biliary system [39, 43]. Aggressive management of biliary obstruction/injury in the setting of postoperative abscess formation is of critical importance to ensure resolution. The risk of complications is minimal with complications such as pneumothorax, empyema, intraperitoneal hemorrhage, and mild pain being the most frequently reported.
Five Key Points on How to Avoid Complications
1.
Assure well-perfused liver remnant following hepatectomy
2.
Assure liver remnant has adequate biliary drainage following hepatectomy
3.
Assure biliary-intestinal anastomoses are well perfused
4.
Assure biliary-intestinal anastomoses are widely patent at the time of surgery
5.
Limit biliary stents as much as possible
Five Separate Key Points on Diagnosing and/or Managing the Complication
1.
Obtain contrasted CT or MRI for unexplained postoperative fever
2.
Utilize interventional radiologic drainage whenever it is technically feasible
3.
Utilize broad-spectrum antibiotics once diagnosed
4.
Utilize surgical approach only for refractory cases
5.
Multidisciplinary team input is critical
References
1.
2.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







