Fig. 11.1
(a) Ultrasonography of colorectal liver metastasis and concomitant liver hemangioma, both hyperechoic without peripheral rim. (b) Surgical specimen: colorectal metastasis and sclerosed hemangioma. H hemangioma, M metastasis
The uncertainty of the diagnosis can be related to atypical imaging findings due to altered morphology or structure or unusual flow patterns [20]. Uncommon enhancement patterns included the so-called flash-filling pattern mostly seen in small hemangioma and marked by intense and uniform enhancement on the arterial phase, hemangioma with perilesional enhancement secondary to arteriovenous shunting, and finally the hyalinized or sclerosed hemangioma with loss of the typical globular enhancement on contrast-enhanced studies related to the replacement of the vascular spaces by hyalinized fibrotic tissue. Sometimes in large hemangiomas, thrombosis of sectorial or lobar portal branch is associated (Fig. 11.2).
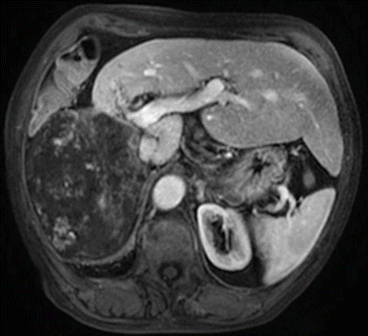

Fig. 11.2
MRI of giant hemangioma of the right liver with thrombosis of the right portal branch and hypertrophy of the left liver
Recently, Portolani et al. [21] analyzed 28 patients with liver cancer who were observed after an initial erroneous diagnosis of hemangioma. The authors reported that the majority of the incorrect diagnoses (75 %) came from low volume center and occurred more frequently with the small nodules in chronic liver disease. For these reasons, as reminded by Duran et al. [22], all cases of hemangioma should be confirmed with at least one MR after 3–6 months, especially in cases of atypical pattern of hemangioma and in chronic liver disease. In situation at risk of having the error (low volume center, poor quality of imaging, etc.), revision of the imaging by an expert radiologist is strongly recommended. On the other hand, it’s also possible to perform an overtreatment of a benign lesion because of a false diagnosis of malignancy. Shimizu et al. [23] reported that hemangioma was the most frequent final diagnosis on 32 patients operated because of a false diagnosis of cancer. In a recent study, the authors showed that T2 shine-through effect is approximately observed in half of the lesions (mainly <1 cm) and in two-thirds of a large patient population with hepatic hemangiomas [22]. Radiologists should be aware of this phenomenon considering that T2 shine-through effect is commonly used to distinguish between benign and malignant lesions. Small solid liver tumors, which eluded preoperative radiological examinations, can be a difficult clinical problem for the surgeon. Intraoperative ultrasonography is crucial for accurate diagnosis [24]. At the intraoperative phase, ultrasonography hemangioma typically appears as a well-defined, lobulated, homogenous hyperechoic mass, but it can be also hypoechoic due to hemorrhage or fibrosis. It is known that small hepatocellular carcinomas and some liver metastases (from colorectal, neuroendocrine, and renal cancers) may also appear as hyperechoic lesions. The problem of differentiating benign from malignant lesions on intraoperative ultrasonography exploration becomes crucial. One useful test for diagnosing a hemangioma in cases of not typical ultrasonographic pattern is the finger compression of suspicious lesions. In fact, when compressed, the hemangioma changes in size and appearance (Fig. 11.3). Nevertheless, in cases of deep lesions or located in the posterior segments, the contrast-enhanced intraoperative ultrasonography provides more useful additional informations. The behavior of lesions during portal and delayed phases has been considered to be helpful in determining the nature of a nodule. The pattern of hypoenhancement in the late phases (washout) is typical of malignancies. In contrast, the benign lesions are isoenhancing or hyperenhancing as hepatic hemangioma [25].
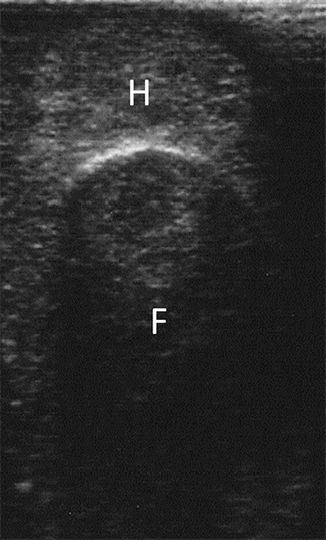

Fig. 11.3
Finger compression maneuver performed to confirm the intraoperative ultrasonography diagnosis of liver hemangioma. F finger, H hemangioma
11.5 Therapy
For most patients, the natural history of hepatic hemangiomas remains uneventful, and surgical intervention can be avoided. There is not much controversy over observing small asymptomatic hemangiomas which are discovered incidentally during a surgical procedure or imaging studies for unrelated problems. Nevertheless, in selected cases an intervention could be indicated, mainly on the basis of symptoms.
11.5.1 Indications for Surgery
Since randomized clinical trials assessing the benefits of surgery are lacking, clear indications for surgical resection in the presence of liver hemangiomas are actually missing, and a treatment algorithm remains controversial. The major indication for surgery is commonly a giant hemangioma causing progressive abdominal pain and patient discomfort. Before considering surgical resection, however, it is mandatory to exclude other possible causes of abdominal complaints, such as gallstones, gastroesophageal reflux disease, or peptic ulcer disease. When the abdominal pain is related to the presence of the hemangioma, still a conservative treatment is justified, even in large tumors, when it is possible to control it with analgesics [26]. In surgical series in which patients were carefully evaluated, 82–100 % were relieved of their complaints after surgery [27–29].
A second reason for considering surgery is the rapid enlargement of the hemangioma. Hemangioma size is usually stable. The increasing in size of the tumor can raise the suspicion of malignancy and should carefully be evaluated [28].
The third most common indication for surgery is diagnostic uncertainty. With the combined use of various imaging techniques, nowadays the diagnosis can be established in most patients. However, exceptions to pathognomonic signs do occur, even if rare. Percutaneous biopsy is considered dangerous because of the risk of bleeding and tumor rupture [30]. Patients who undergo surgery to exclude malignancy are more likely to be male, asymptomatic, have a history of cancer, and have smaller lesions [31].
Kasabach-Merritt syndrome requires a surgical intervention, but its incidence is extremely rare. Emergency surgery for complicated liver hemangiomas is seldom necessary, since bleeding, spontaneous rupture, and tumor thrombosis have been exceptionally reported [32].
Some authors support operative treatment for patients with large hemangiomas, mainly in the left liver, whose hobby or occupation carry a risk of hepatic trauma. This prophylactic indication has never been established, but it seems reasonable for the safety of the patient [28]. An interesting study compared resection versus conservative management of liver hemangiomas [33]: the rate of adverse events was similar (14 % of perioperative complications versus 20 % of persistent or new onset of hemangioma-associated symptoms) with an overall low risk for potentially life-threatening complications (7 % versus 2 %). These results could justify surgical indication for patients with giant hemangiomas carrying the risk of trauma.
Patient anxiety and his willingness because of the undesirable feeling of living with a hepatic tumor, even if asymptomatic, should not be considered a good indication for surgery anymore [18]. Even if the patient’s wish has to be considered, the surgeon must well explain the risk of liver surgery comparing to the benign natural history of the disease.
During the last 20 years, there has been an increase up to 26 % in laparoscopic procedures for benign tumors of the liver, including hemangiomas [34]. In the present era of spread of mini-invasive liver resections, the above indications should be maintained and cannot be widened by the possible laparoscopic approach.
11.5.2 Surgical Technique
When indicated, liver hemangiomas can be resected with low morbidity and minimal mortality rates (Table 11.1). The first step of the operation should always be an intraoperative ultrasonography in order to assess the tumor relationships with portal pedicles and hepatic veins and to identify small hemangiomas that could be included in the resection plane. Hepatic pedicle clamping can be particularly useful in order to reduce the size of the tumor and to facilitate the manipulation of the tumor and the mobilization of the liver. Selective hepatic vascular exclusion, maintaining the caval flow, has been reported to be safer in patients with giant hemangiomas compressing the major hepatic veins [35].
Table 11.1
Surgical series of resected liver hemangiomas
Author | Year | # Observed | # Operation | Median size | Enucleation (%) | Morbidity | Mortality |
|---|---|---|---|---|---|---|---|
Schwartz | 1987 | 28 | 16 | 10 | 2 (13) | NR | 0 |
Iwatsuki | 1988 | 411 | 100 | 12 | 0 | NR | NR |
Baer | 1992 | NR | 10 | 14.5 | 10 (100) | 1 | 0 |
Belli | 1992 | NR | 24 | 11 | 0 | 2 | 0 |
Petri | 1993 | NR | 51 | 5.6 | 29 (51) | 13 | 0 |
Brouwers | 1997 | NR | 24 | 11 | 0 | 5 | 0 |
Weimann | 1997 | 238 | 69 | 8.3 | 26 (38) | 13 | 0 |
Ozden | 2000 | 171 | 39 | 10 | 33 (85) | 5 | 1 |
Reddy | 2001 | 71
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



