Fig. 10.1
Various tip shapes of guidewires. From [6], reprinted with permission from Elsevier Limited
Wire Rigidity
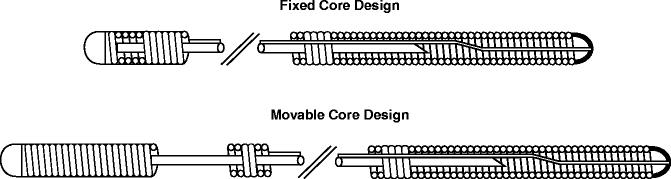
Urological guidewire shafts are composed of a solid metal core surrounded by a steel outer coil (Fig. 10.2). The core is made of either steel or nitinol, a metal alloy made of nickel and titanium that allows for shape memory and superelasticity while providing columnar strength to resist kinking. The core diameter determines the stiffness of the wire. Increased shaft stiffness is useful for straightening tortuous anatomy and serving as a working wire in cases where a routine guidewire is inadequate. The wire core is tapered as it approaches the tip, and the length and degree of tapering determine the tip flexibility; moveable core wires are available, where the length of the flexible tip can be adjusted simply by advancing or withdrawing the mandrel. The outer coil can either be flat or round; flat coils allow for a smoother outer surface and can accommodate in a larger diameter inner core. Among the stiffest guidewires in urological use, the Amplatz Super Stiff Guidewire (Boston Scientific, Natick, MA) features a flat outer coil for increased stiffness, thus providing stability within the urinary tract without compromising tip flexibility.
Surface Coating
Guidewires are coated with various substances that serve to decrease friction when passing instruments over them and/or to facilitate access into tortuous or obstructed ureters. Surface coatings include polytetrafluoroethylene (PTFE) or hydrophilic polymers. Most guidewires, including the Amplatz Super Stiff, Sensor, and Zebra hybrid wire (nitinol wire with striped PTFE jacket for increased torqueability; all Boston Scientific, Natick, MA), utilize a PTFE coating. The Roadrunner (Cook Urological, Spencer, IN) and Glidewire (Terumo Medical, Tokyo, Japan) feature a malleable nitinol core with hydrophilic coating and soft, floppy tip that provides a negligible coefficient of friction, thus making them useful for navigation beyond areas of impaction, stricture, or tortuosity. These wires can become quite slippery due to their hydrophilic nature, and thus should not be utilized as a safety wire. If access is achieved with a hydrophilic wire, it should be exchanged, if possible, for a PTFE-coated wire in order to prevent loss of access. A torque device can be used with hydrophilic wires for added control and assistance with maneuvering the wire into its desired position; if a torque device is not readily available, a loop can be made in the distal portion of the wire for better grasping and torque. Finally, dry gauze can be wrapped around the wire and manipulated by the surgeon to achieve the same result.
Comparison of Guidewires
Given the number of options available, intimate knowledge of the above properties becomes essential in selecting the appropriate wire for a given situation. The ideal guidewire should be flexible enough to bypass any point of obstruction, firm enough to secure access in the desired kidney, rigid enough to permit passage of instruments, and soft enough to do all of the above without traumatizing the urinary tract.
In attempt to predict their clinical performance, Sarkissian et al. analyzed the physical and mechanical properties of five commonly used guidewires: U-Nite (Bard Urological, Convington, GA), Sensor (Boston Scientific), Amplatz Superstiff (Boston Scientific), NiCore (Bard Urological), and RadiFocus glidewire (Boston Scientific). The primary aim was to compare their stiffness to a reference standard, the Amplatz SuperStiff wire. Secondarily, they compared the hydrophilic tips against the NiCore and the RadiFocus glidewires. Tests performed included measurement of coefficient of friction, shaft buckling force, tip bending force, and tip puncture force.
The Sensor wire was found to have the greatest coefficient of friction, while the U-Nite wire was found to have the lowest, with a maximum extraction force up to 0.1 N less than the reference standard. Shaft buckling measurements demonstrated that the Amplatz SuperStiff, as expected, had at least a 34.3% greater shaft buckling force compared to all other wires. The NiCore guidewire was found to have a significantly stronger tip than all of the other guidewires tested, with a 62% greater force than the RadiFocus, Sensor, and Amplatz SuperStiff, and up to a 47.6% greater force than the U-Nite guidewire. Finally, the Sensor wire required the least amount of force to puncture a standard 0.016 mm sheet of tin foil, while the RadiFocus required a minimum of 14.9%, 19.0%, 33.9%, and 47.9% greater force than the Amplatz SuperStiff, NiCore, U-Nite, and Sensor wires, respectively. The authors conclude from this work that these in vitro studies can be used to guide decision making in the operating room. The Amplatz SuperStiff wire remained the stiffest wire and was determined to be the best suited for placement of access sheaths and large stents, while all of the Boston Scientific Wires required less force than the Bard wires to bend around a point of obstruction [7].
In a similar in vitro trial, Clayman et al. compared tip bending force, shaft bending force, pull force, and tip puncture force of the UroWire XF, Amplatz, and Bentson PTFE wires (All Applied Medical, Rancho Santa Margarita, CA); PTFE Guidewire (Bard); Glidewire, Amplatz Super Stiff, and Bentson guidewire (Boston Scientific); and PTFE Wire Guide, and Roadrunner (Cook urological). Featuring a 15 cm floppy tip, the Boston Scientific PTFE guidewire required the least amount of force to bend the tip. Of the wires with a 3 cm tip, the Applied Bentson wire was the most flexible, and there was no difference in tip flexibility between the Applied Medical or Boston Scientific brands of Amplatz wires, and there was no difference in tip flexibility among the hydrophilic wires. With regard to lubricity, the Boston Scientific glidewire required the lowest withdrawal force of all wires, and was found to have significantly less withdrawal force than the Applied Urowire (p = 0.002) but not significantly less than the Cook Roadrunner (p = 0.155). The Boston Scientific Amplatz wire required the greatest shaft bending force compared to all other wires (p < 0.05), while all of the nitinol hydrophilic wires appeared to be equally flexible. Finally, with regard to tip safety, the Boston Scientific glidewire required four times more force to puncture a sheet of aluminum foil than all of the other wires (p < 0.001), making it the safest wire in use [8].
A third comparative trial was performed by Liguori et al., who measured in vitro tip buckling, resistance to bending, and force at which permanent plastic deformation occurred. Fresh human cadaveric ureters were then used to determine force required to perforate a ureter and frictional coeffecient while traversing the ureter. Wires tested included the Boston Scientific PTFE guidewire, Radiofocus Guidewire M (Terumo Europe N.V., Leuven, Belgium), Medtronic PTFE guidewire (Medtronic, Danvers, MA), Emerald Guidewire (Cordis, Miami, FL), and Sensor Dual Flex wire (Boston Scientific). As expected, the wires with hydrophilic tips (Sensor Dual Flex and Radiofocus) required the least amount of bending force, while the Medtronic PTFE wire had the stiffest wire tip. The Boston Scientific PTFE wire was the most resistant to bending while the Terumo Radiofocus wire had the most flexible shaft. The Cordis Emerald and Medtronic PTFE wires were most easily deformed, while the Boston Scientific PTFE guidewire required the greatest force applied before undergoing plastic deformation. Both hydrophilic wires could not be deformed. The Medtronic PTFE wire required the least force to perforate the cadaveric ureter (less than 2.5 N), followed by the Boston Scientific PTFE and Cordis PTFE wires; both hydrophilic wires did not perforate the ureter during testing. This use of cadaveric ureter was validated in the same study as all five wires were tested for their ability to perforate paraffin film. The same results were seen, with the Medtronic wire requiring the least force, followed by the Boston Scientific and Cordis wires; in this test, the Terumo wire was able to puncture the paraffin wax, albeit at a much greater force. Finally, in the test of lubricity, the hydrophilic wires required the least withdrawal force, while the Cordis PTFE wire demonstrated the greatest force of friction [9].
Patriciu and colleagues, in an attempt to more closely estimate the forces applied by wires to anatomic structures, created a biomedical model composed of thermoplastic rubber, motion sensors, charge-coupled-device camera, computer, pneumatic grippers for wire control, and linear/torsional/axial force detectors (Fig. 10.3). Wires were tested on a model of a straight ureter, tortuous path, and a ureter obstructed with a 5.5 mm simulated stone. Calculations were made both for simple wire passage as well as for passage of a catheter over each wire. Wires tested included the Hydro-Glide (Bard); Glidewire, Lubriglide, and Sensor (all Boston Scientific); and Hiwire and PTFE guidewire (both Cook). Catheters tested were a 6 Fr Open-ended tapered tip catheter (Cook) and a prototype everting film catheter (Percutaneous Systems, Inc.) The authors found that the lowest forces recorded for passage along a straight path were with the Hydro-Glide. The Lubriglide wire demonstrated the lowest axial forces when placed on the tortuous and obstructed ureter models, while the Cook PTFE guidewire demonstrated the lowest transversal force applied to the ureteral walls. For all catheter placement tests, the everting film catheter demonstrated lower forces than the Cook catheter [10].
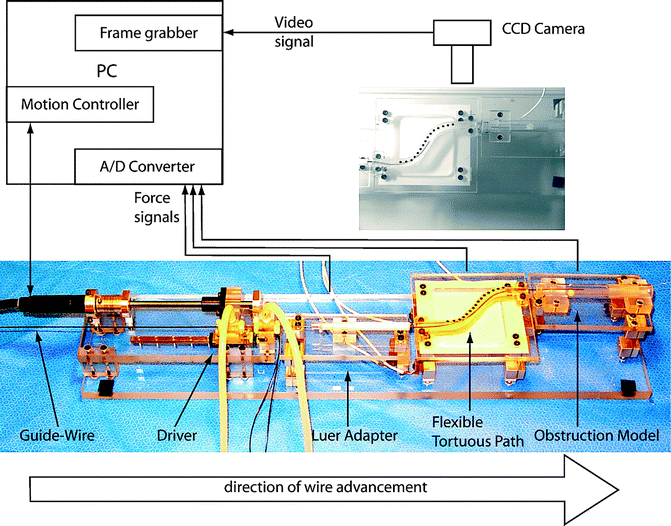

Fig. 10.3
Biomedical model for testing wires. From [10], reprinted with permission from Elsevier Limited
From all of these comparative studies on guidewire use in Urology, it is evident that the wire selected for a given procedure should provide the strength required to secure access, yet it should have a large “margin of error”—the difference between the force required to perforate a ureter and the force required to bend the tip [11]. As technology continues to evolve, it is imperative that continued product testing be performed to ensure that indications for use are current, safety standards are met, and techniques continue to evolve with novel product designs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree