Gastrointestinal Stenting: Indications and Techniques
ESOPHAGEAL STENTING
The majority of esophageal stents in our practice are inserted for palliation of malignant dysphagia caused by esophageal carcinoma (Fig. 2–1) or by extrinsic compression from malignant lymph nodes or masses. Stents may also be used to palliate tumor recurrence at surgical anastomoses, and we have occasionally had to resort to the use of stents in the treatment of benign strictures that responded poorly to balloon dilatation. Tracheoesophageal fistulae and esophageal leaks can also be successfully treated with covered stents.
Esophageal carcinoma is a relatively common disease with a poor prognosis, accounting for 3500 deaths per year in the United Kingdom1 and ranking as the seventh most common malignancy world-wide.2 At the time of presentation, 75% of patients have disease spread to lymph nodes,3 and approximately 50 to 60% of patients are not suitable for attempted curative surgical resection. Available palliative treatments include surgery, radiotherapy, chemotherapy, use of rigid plastic tubes, laser therapy, and the use of self-expanding metallic esophageal stents.
Stent Types
A variety of covered and uncovered stents are available. The three most commonly used are the Wallstent (Schneider AG, Zurich, Switzerland), the Strecker stent (Boston Scientific Corporation, Watertown, MA, USA), and the Gianturco stent (William Cook Europe, Bjaeverskov, Denmark).
The Wallstent is made from a stainless steel alloy tubular mesh available in its covered form in two sizes for use in the esophagus: 20 mm diameter and 110 mm length, or 25 mm diameter and 105 mm length. The most commonly used Wallstents have a polyurethane coating on the outer surface, except for 15 mm at each end, though an uncovered esophageal Wallstent is also available. The delivery system consists of three coaxially arranged shafts in which the compressed stent is mounted. This arrangement allows partial release of the distal half of the stent, with the facility to reposition it prior to full deployment, either by recovering the stent and moving it to a more distal position, or by leaving the distal end partially deployed and gradually withdrawing the delivery system to the desired point. Wallstents with an outer polyurethane covering have been shown to have a high migration rate, especially when used to treat low esophageal strictures where the stent has to be placed across the gastroesophageal junction. They are now being superseded by a new design, which is conical and has an inner polyurethane covering, allowing the mesh of the stent to come directly into contact with the esophageal mucosa, thus increasing friction.
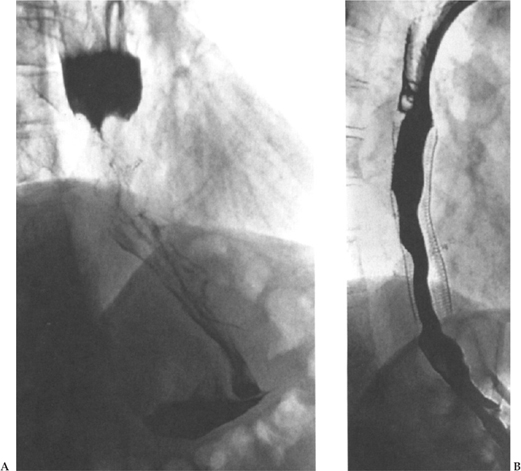
Figure 2–1 (A) Contrast esophagogram demonstrating a tight stricture at the esophagogastric junction due to an adenocarcinoma. (B) After insertion of a Strecker stent, barium is seen to flow freely into the stomach. This patient experienced excellent palliation of dysphagia and was able to tolerate a normal diet.
The uncovered Strecker stent is made from knitted nitinol, an alloy with thermal memory characteristics. It is available in a single diameter (18 mm), with lengths of 7, 10, or 15 cm. Until recently, the stent has been compressed onto the delivery system encased in gelatin, which dissolves after retraction of the outer sheath, thus allowing the stent to deploy. It is important to leave the delivery system in situ for several minutes to allow dissolution of the gelatin to occur because any early attempt at removal of the delivery system prior to adequate stent expansion is likely to lead to displacement or buckling of the endoprosthesis. This system can create difficulties when sedation has been less than adequate and the patient is restless. Recently, a covered Strecker stent became available; this one is compressed onto the shaft of the delivery system by a thick woven silk suture. When the stent has been correctly positioned, the suture is pulled, thus unraveling, and the stent is progressively released. This permits quicker deployment and gives the capacity for some adjustment to stent position after initial partial distal release. The Strecker esophageal stent provides a lower degree of outward radial force than the other stents that are available.
The Gianturco stent is composed of 2-cm-long basic units made from 0.018-in. stainless steel wire bent in a zig-zag pattern. The units are sutured together to form prostheses ranging in length from 4.5 to 18 cm. Various modifications on this basic design are available, but all are covered with polyurethane and have barbs to prevent migration.
The choice of stent depends to some extent on personal preference, though, as will be described later, the different stents available have certain advantages and disadvantages that have been demonstrated in a number of studies.
Technique of Insertion
An initial contrast swallow is performed to delineate the site and length of the lesion to be treated (Fig. 2–2A). At this point the size of stent required and/or the necessity for the use of more than one stent can be decided. After obtaining informed consent, the patient lies on the fluoroscopic table in the left lateral position. Xylocaine spray is applied to the pharynx, and the patient is sedated with an intravenous agent such as midazolam. Suitable catheters and guide wires are used to cross the stricture (Fig. 2–2B). In very tight strictures or in cases of esophageal occlusion, hydrophilic guide wires are valuable. The catheter and guide wire are manipulated into the duodenum to provide as stable a position as possible, and the guide wire is exchanged for an Amplatz stiff exchange wire. A 15-mm-diameter balloon is used to predilate the stricture prior to stent deployment (Fig. 2–2C). After dilatation the stent is deployed according to the technique for that particular system (Fig. 2–2D). Due to its weaker radial force the Strecker stent often requires additonal balloon dilatation. We aim to deploy the stent with approximately 60% of its length above the middle of the stricture in an effort to minimize the incidence of distal stent migration. Long strictures may require the use of more than one overlapping stent.
Immediately after the procedure, nonionic contrast medium is introduced into the esophagus via the catheter to expose procedural complications, especially esophageal perforation, and to confirm stent patency. Patients remain in hospital overnight, and once they have recovered from the effects of the sedation, they are allowed to take small volumes of clear fluids orally. The following day another contrast esophagogram is obtained to determine if further intervention is required. For example, a stent may show persistent narrowing, requiring balloon dilatation, or there may have been migration, requiring the insertion of an additional stent coaxially within the first endoprosthesis and overlapping with it to prevent further slippage. If the esophagogram shows good stent position and function, then patients are allowed a normal diet. They are advised to cut their food into small pieces, to chew it thoroughly, and to have carbonated beverages after each meal to clear the stent of any food debris.
Any patient in whom a stent has been placed across the gastroesophageal junction will eventually experience reflux of gastric contents. The symptoms are controlled by the administration of omeprazole (a suppressor of gastric acid secretion), which is routinely started after the procedure.
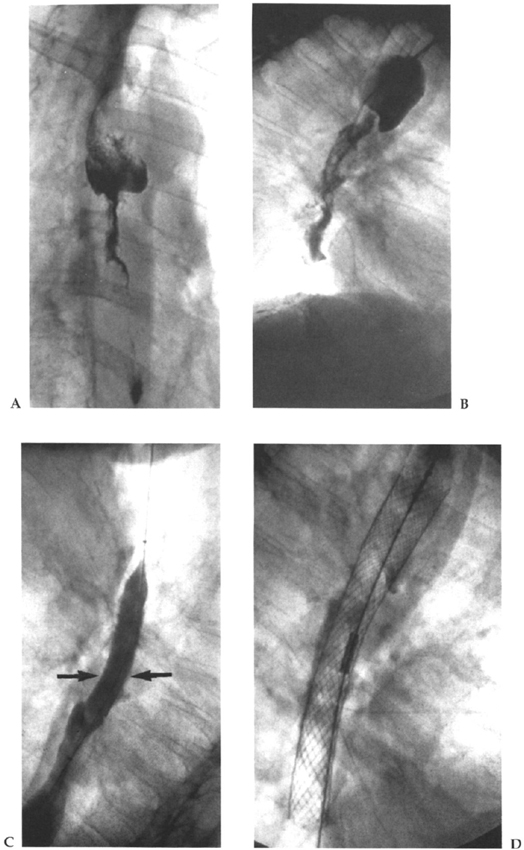
Figure 2–2 (A) Contrast esophagogram showing a long esophageal stricture caused by squamous carcinoma. (B) A catheter has been inserted into the esophagus part way along the stricture and contrast fluid injected to delineate the lesion. (C) The stricture is predilated with a 15-mm-diameter esophageal balloon (arrows). (D) Appearance immediately after deployment of a 20-mm-diameter Wallstent.
| Dysphagia Score | Degree of Dysphagia |
|---|---|
| 0 | No dysphagia |
| 1 | Able to swallow semisolid food only |
| 2 | Able to swallow liquids only |
| 3 | Difficulty in swallowing liquids and saliva |
| 4 | Complete dysphagia |
Results of Esophageal Stenting in Malignant Disease
The degree of dysphagia can be assessed by the use of a dysphagia score, where 0 is equivalent to no dysphagia and 4 indicates total dysphagia (Table 2–1). The overall results of six recently published series are summarized in Table 2–2.4–10 All of the procedures were conducted without general anesthesia and were technically successful, though there were some instances of initial stent misplacement requiring deployment of a second device. Improvement in the dysphagia score can be expected in 83 to 100% of patients when they are assessed 24 hours after the procedure.4–9 Our experience at St. George’s Hospital, for example, showed improvement from a mean dysphagia score of 2.6 prior to stenting to 0.5 after stent placement.9
The difference in the stent designs means that they are susceptible to different types of complications. Thus, the Strecker stent with its weaker radial force often requires additional postdeployment balloon dilatation. In addition, the Strecker stent is too weak to be used in cases of malignant dysphagia due to extrinsic compression. Its use in this situation has been reported to be associated with recurrence of dysphagia due to stent collapse.9 Therefore, the use of the stronger Wallstent is recommended in cases of dysphagia due to extrinsic compression. Uncovered Strecker stents are prone to tumor ingrowth (20 to 30%). The lack of an outer covering probably also accounts for the absence of migration with this type of stent.
Both Wallstents and Gianturco stents are associated with an incidence of 1.5 to 15% of delayed upper GI hemorrhage. In the Guy’s Hospital experience, autopsies were performed on the patients who died from hemorrhage, and no causal relationship between the stent and bleeding could be found. Other complications include severe pain, aspiration pneumonia, fistula formation (Fig. 2–3), and stent migration. The latter complication occurs in 30% of covered Wall stents and 10 to 15% of Gianturco stents. Covered stents are particularly liable to migrate when positioned at the gastroesophageal junction, with a free end lying in the stomach. The essential factor appears to be the stent covering, which reduces the friction between the esophageal wall and the stent. Wallstents rely on the uncovered portion at either end to prevent migration, and if one end lies free in the stomach this mechanism is compromised. Tumor ingrowth is seen in up to 2% of patients treated with covered stents (Fig. 2–4). In cases of complete and symptomatic stent migration, the stent can be removed by surgical gastrotomy. In cases of partial migration, the covered stent can be stabilized by the placement of a second uncovered coaxial stent through its proximal end. Because of the propensity of covered stents placed over the gastroesophageal junction to migrate, we now elect to deploy uncovered stents proximally at this site, although as new Wallstent designs become available our practice may change. For example, early experience suggests that a conical design may reduce the incidence of distal migration, though in a pilot study we found that proximal migration occurred in 2 of 10 conical stents.
Recurrence of dysphagia secondary to tumor ingrowth or overgrowth can be successfully managed either with endoscopic laser therapy or by placement of additional stents. Food bolus impaction is easily cleared endoscopically.
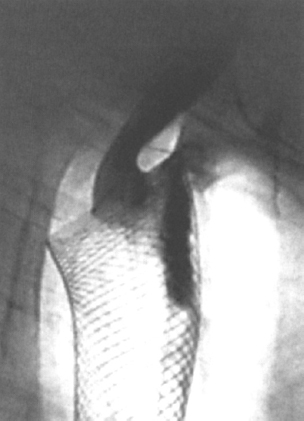
Figure 2–3 Tracheoesophageal fistula related to the upper end of a Wallstent that had been in situ for 3 months. There was no evidence that this was due to tumor, and it is presumed to have been caused by pressure necrosis.
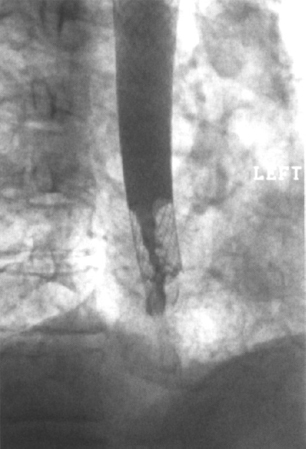
Figure 2–4 Patient had developed recurrent dysphagia 9 weeks after insertion of a covered Wall stent. Contrast esophagography demonstrated tumor ingrowth into the uncovered section at the lower end of the stent. The covered portion of the stent remains unaffected.
Comparison with Other Techniques
Conventional palliative therapy for advanced esophageal carcinoma includes surgery, radiotherapy, and chemotherapy. Palliative surgical resection is highly invasive and is associated with a high operative mortality and morbidity.11–14 External-beam radiotherapy improves dysphagia in around 50% of patients, but at the expense of fibrotic stricutre formation in approximately 30%.15–19 Intracavitary brachytherapy, either alone or in combination with external-beam radiotherapy, gives better results20–22 but has the major drawback of causing esophagitis in up to 80% of patients.22,23 Combined chemotherapy and radiotherapy also produce improved results, but with greater morbidity.24–28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








