Gastrointestinal Cancer: Screening and Surveillance
Robert S. Sandler
Screening can be defined as the application of a test to detect a potential disease or condition in a person who has no known signs or symptoms (1). A screening test need not provide a definitive diagnosis, and most screening tests are not definitive. Instead, they help stratify screened subjects into those who are likely and those who are unlikely to have disease. Those at higher risk are then candidates for further diagnostic testing. Screening can be conducted by asking questions (e.g., about family history of cancer), by performing a physical examination (e.g., digital rectal examination), by obtaining a laboratory test (e.g., α-fetoprotein), or by performing an imaging or endoscopic examination. Ideally, a screening test should be cheap, safe, quick, well accepted, and well tolerated.
Surveillance is a special case of screening. It involves periodic reapplication of a screening or diagnostic test. Surveillance, too, may be restricted to those at highest risk, but it does not have to be. In contrast to screening, which detects existing or prevalent cases, surveillance is designed to detect new or incident cases.
This chapter reviews the general principles of screening and surveillance for gastrointestinal (GI) cancers. These principles are illustrated with specific examples, but detailed discussions of screening for individual cancers can be found in other chapters.
General Principles of Screening
Disease Must Have Serious Consequences
The first general principle of screening is that the disease must have serious consequences (2). Diseases that have significant morbidity and mortality would be candidates for screening. Importantly, the disease must also be recognized as serious by potential screenees for them to agree to participate in a screening program. Some conditions that demand screening are not themselves serious but lead to serious diseases. Barrett’s esophagus, for example, is a benign condition that predisposes to adenocarcinoma of the esophagus. Routine surveillance of patients with Barrett’s esophagus may decrease mortality from esophageal carcinoma. To achieve success, a Barrett’s esophagus screening program requires that patients recognize the connection to cancer and embrace regular surveillance.
Disease Must Be an Important Health Problem
The disease must be an important health problem (3) to justify screening. This requirement has both societal and practical implications. From a public health or population perspective, allocating resources for population screening is not justified unless the disease is relatively common. In practical terms, if the disease is uncommon, large numbers of asymptomatic individuals will be subjected to screening with a very small yield. Thus, a highly fatal disease such as cholangiocarcinoma would not be a candidate for screening in the general population because it is too rare.
The “importance” requirement is dynamic and may vary with geography. Gastric cancer screening has been promoted in Japan, where disease incidence is high, but screening does not appear to be effective in Venezuela (4). High-risk subpopulations who would be candidates for screening for gastric cancer may also exist. Individuals who have had previous gastric resection for benign peptic ulcer disease are at higher risk of developing cancer in the gastric remnant after a 15- to 20-year latency (5). Such individuals might be candidates for screening in Germany, where the baseline risk of gastric cancer in the general population is moderate, but not in the United States, where the baseline risk is low.
The disease may be important to special populations. Although screening of the entire population might not be appropriate, screening might be very appropriate in a high-risk subgroup. An example would be screening for hepatocellular carcinoma. Screening is not recommended for the general population in the United States but may be appropriate for certain patients with cirrhosis.
Disease Must Have a Detectable Preclinical Phase
The purpose of screening is to detect cancer before symptoms develop. This means that the cancer must have a presymptomatic or preclinical phase during which it is potentially detectable by a screening test (3). For benefit to be derived from screening, it must be possible not only to detect the cancer during the preclinical phase but also, more importantly, to alter the natural history. The natural history of cancer is represented in Figure 4.1.
The biological onset of cancer occurs at point A in Figure 4.1. No units of measure are given in the figure, and the intervals are all relative. The age of development of cancer varies by cancer and may be modified by environmental and familial risks. For example, cancers secondary to radiation or gene mutations (e.g., familial polyposis) occur at a younger age than sporadic cancers.
At the time of onset (A), the tumor is too small to be detected. The point at which the disease is first potentially detectable by screening is shown as point B. The location of B depends on the tumor type and growth potential. For tumors
that grow rapidly, the interval between A and B is short. The interval between B, the point at which the tumor is first potentially detectable by screening, and C, the point at which symptoms become apparent, is termed the detectable preclinical phase (6). Unless a preclinical phase exists during which the cancer is potentially detectable, no rationale exists for screening. The point in the detectable preclinical phase when the cancer is detected depends on the screening test; a more sensitive screening test has the potential to detect disease earlier in its natural history. An endoscopic procedure, for example, has the potential to detect a colorectal adenoma earlier than a fecal occult blood test. The detectable point also depends on characteristics of the disease. Colorectal cancers that do not bleed cannot be detected by fecal occult blood tests.
that grow rapidly, the interval between A and B is short. The interval between B, the point at which the tumor is first potentially detectable by screening, and C, the point at which symptoms become apparent, is termed the detectable preclinical phase (6). Unless a preclinical phase exists during which the cancer is potentially detectable, no rationale exists for screening. The point in the detectable preclinical phase when the cancer is detected depends on the screening test; a more sensitive screening test has the potential to detect disease earlier in its natural history. An endoscopic procedure, for example, has the potential to detect a colorectal adenoma earlier than a fecal occult blood test. The detectable point also depends on characteristics of the disease. Colorectal cancers that do not bleed cannot be detected by fecal occult blood tests.
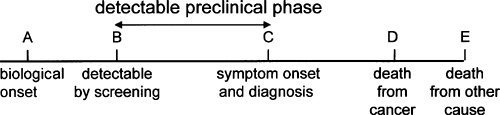 FIGURE 4.1. Natural history of cancer. A screening test must be able to detect cancer during the preclinical phase of disease. |
Symptom onset is depicted in Figure 4.1 at point C. The point of symptom onset represents the point of usual diagnosis in the absence of screening. For simplicity, the time of symptom onset and the time of usual diagnosis are represented as a single point in Figure 4.1, but the time of symptom onset and time of diagnosis are not always identical. A delay may occur before the individual first seeks medical attention after the onset of symptoms (patient delay). For example, patients may ignore rectal bleeding and thereby delay the diagnosis of rectal cancer. Even after the patient presents to the physician with symptoms, further delay may occur before the diagnosis is made (physician delay). The interval between presentation and diagnosis may be very brief if definitive tests are scheduled promptly or may be longer if symptoms are dismissed by patients or physicians. If the presenting symptoms are nonspecific or vague, such as nausea, fatigue, or weight loss, diagnosis may be substantially delayed.
After disease diagnosis and therapy, the patient may die from disease (D) or may be cured to later die from some other cause (E).
Treatment Must Exist That Is More Effective When Applied to Presymptomatic Cancer
An effective treatment for the disease must exist to justify screening. Moreover, the treatment must be more effective when it is applied during the presymptomatic interval than when it is applied to cancers detected at the usual time of symptom onset. Otherwise, there is no point in attempting to detect the cancer earlier. Determining whether early detection has a real impact in prolonging life is also important.
As shown in Figure 4.1, if a lesion is discovered during the preclinical phase, then the interval between diagnosis by screening and death (B→D) is longer than the interval between diagnosis after symptom onset and death (C→D). Death occurs at the same point, however. This apparent increase in survival is termed lead-time bias. Early detection has simply advanced the date of diagnosis without postponing death. A screening test is useful only if detection during the preclinical phase postpones death and prolongs life.
The survival advantage from early detection is D→E, the interval between the time of death from the cancer that was averted and the time of death from another cause. The age at death from competing cause is variable and unpredictable. Clearly, the potential years of life saved by screening are greater for a 40-year-old than for an 80-year-old because of the longer life expectancy of a 40-year-old. The years of life saved are also fewer for individuals with significant comorbidity. Because death from competing cause is unpredictable, specifying when not to screen has been difficult for guideline developers. Clinicians often exercise their judgment by not screening the very elderly or those with substantial comorbidity. The overall effectiveness of a screening program depends on the number of life-years gained by screenees.
The point of death from other cause (E) can precede the point of death from cancer. Some patients die with their cancer but not from their cancer. If the cancer is not likely to be fatal, then detecting the cancer by screening incurs costs without prolonging life.
Test Must Be Safe
Screening tests are generally applied to individuals at low risk for cancer. Colorectal cancer screening, for example, is recommended for asymptomatic individuals older than 50 years. Because the risk of the disease is low, the screening test must be exceptionally safe. Otherwise, the risk of the screening test outweighs the benefits. The mortality from complications of the test must certainly be lower than the mortality from the disease.
Some authorities have recommended periodic colonoscopy to screen for colorectal cancer (7). Data from prospective studies of colonoscopy suggest that approximately 1 per 1,000 persons undergoing colonoscopy have perforations, 3 per 1,000 have major hemorrhage, and 1 to 3 per 10,000 die as a result of the procedure (8). Although these risk estimates probably overstate the risk in asymptomatic 50-year-old screenees, the risks of the procedure must be balanced against the potential gains.
The acceptable risk from screening depends on the population. It may be reasonable to adopt a riskier screening test if the risk for disease in a particular group is very high with the expectation that the risk–benefit ratio will remain favorable. Thus, although colonoscopy might be viewed as possibly less safe for asymptomatic 50-year-olds, the test would be quite appropriate for a member of a family with hereditary nonpolyposis colorectal cancer in whom the risk for colon cancer is substantial (8).
The issue of safety must extend beyond the specific screening test to the more definitive diagnostic tests that are applied to individuals for whom screen results are positive. Individuals who are found to have positive results on a fecal occult blood test are referred for colonoscopy, a more definitive but riskier test (9). The risk for colonoscopy must be incorporated into risk estimates for a fecal occult blood test program. To do otherwise would falsely inflate the safety of the fecal occult blood test. For example, in the Minnesota fecal occult blood trial, 12,246 colonoscopies were performed at the university hospital in subjects with positive fecal occult blood test results (10). The colonoscopies resulted in 4 colonic perforations requiring surgery and 11 serious bleeds, 3 of which required surgery. Decision and cost-effectiveness models must incorporate these downstream risks when they evaluate a screening program.
Test Must Be Acceptable to Patients and Providers
To be effective, a screening test must be acceptable not only to potential screenees but also to their health care providers.
Lack of acceptance by either of these groups compromises the effectiveness of a screening program.
Lack of acceptance by either of these groups compromises the effectiveness of a screening program.
Acceptability to potential screenees is highly personal and largely unstudied (11). Fecal occult blood testing is touted as a simple method for mass screening, yet compliance in European controlled trials ranged from 53% to 67%. Fecal occult blood testing has been offered in Germany since 1977, but only 21% of women and 10% of men take advantage of the screening (12). Flexible sigmoidoscopy, a test that many believe to be more effective than mammography, is vastly underused. Lack of acceptance of sigmoidoscopy is largely due to the failure of patients to embrace the technique, either because they perceive that the test is uncomfortable or because they believe that they are not at risk. Providers share part of the responsibility, however. Many do not have the time, training, or equipment to provide flexible sigmoidoscopy. The use of nurse practitioners may help make the test acceptable to health care providers and thereby more available to patients (13,14). New technology, such as a sheathed endoscope that does not require time-consuming disinfection between uses, may also make a test more acceptable to providers by decreasing the time necessary to perform the test, although this technology has not become popular (15).
Test Must Be Available
The screening test must be available to potential screenees to have an impact. As noted previously, flexible sigmoidoscopy is not always available. Availability must also extend beyond the screening test to diagnostic tests for those who have positive results on the screening test. If colonoscopy is not available to evaluate individuals with positive fecal occult blood tests, then the program (screening test plus diagnostic test) is not available. Virtual colonoscopy is a radiologic technique that holds great promise for large bowel screening (16). It may take some time before the technology is widely available.
Test Must Be Affordable
The screening test must be reasonably inexpensive in both direct costs (dollars) and indirect costs (time missed from usual activities, discomfort, and inconvenience). The cost of the test to potential screenees depends, in part, on whether the test is covered by insurance. In 1998, Medicare began to cover the cost of screening with fecal occult blood tests every year and with flexible sigmoidoscopy every 2 years for individuals older than 50 years. Coverage by Medicare made the test more affordable, but it is probably too soon to determine whether any impact on use has occurred.
The cost of a test includes the cost of the test itself and any downstream costs for more definitive tests that are necessary to evaluate a positive test result. For example, fecal occult blood test kits are inexpensive (<$10), but the cost to evaluate a positive test may be $1,000. The cost of a screening program also includes the cost of treatment for lesions that are discovered as a consequence of screening. The cost of a colorectal cancer screening program includes the costs of screening (endoscopy and pathology) and the costs of treatment (surgery, oncology, radiation). The costs of the screening program must be balanced against the gains (17). Early detection should prolong life. An affordable program should have a reasonable cost per life-year gained when compared with other commonly accepted medical interventions (18).
The affordability criterion depends on perspective. Policy makers typically take the population perspective in deciding whether a screening test should be offered. To reach a decision, they evaluate the overall costs to society of the screening test and the opportunity costs of not spending the money on a different program. The population perspective is often at odds with the perspective of the individual patient, who may have the interest in and the funds to purchase the test, and the perspective of the physician, who has the obligation to serve as the advocate for the individual patient.
Test Must Be Accurate
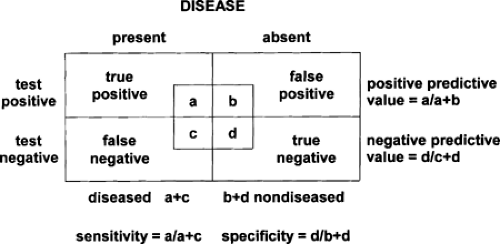
Perhaps the most important requirement for a screening test is accuracy. To have an impact, the test must have high sensitivity and specificity to avoid misclassification. These concepts are discussed in the next section.
Characteristics of the Screening Test
Sensitivity
The fundamental purpose of screening is to distinguish people who potentially have the disease of interest from those who do not. The ability of a screening test to designate those with disease as positive is referred to as the sensitivity of the test (19). Sensitivity is defined as the proportion of diseased individuals with a positive test result (Fig. 4.2). A sensitive test has few false-negative results. Therefore, when a sensitive test is negative, it helps to rule out disease. That is because a sensitive test rarely gives negative results in the presence of disease (few false negatives) (20). A sensitive test should be used if an important penalty exists for missing disease.
The sensitivity of a test to detect disease may vary with the stage of disease. For example, an upper GI tract radiograph is more sensitive for a 10-cm mass than for a 1-cm mass. When tests are first developed, they are often used to evaluate patients with symptomatic or advanced disease. A test that is highly sensitive under these circumstances may perform more poorly for asymptomatic early-stage cancer (21). For a screening test to be effective, it must detect the disease in the preclinical phase and must have an impact on survival. A test that is only sensitive when the disease is advanced is not useful.
For tests such as fecal occult blood testing, a family of test sensitivities may exist. Each fecal occult blood card consists of two testing windows, and testing generally involves three cards (six windows). Testing may be repeated yearly. Thus, there is the sensitivity of each slide, the sensitivity of the set of three slides, and the sensitivity of the program repeated over years. These sensitivities are distinct but related (22). Different authors have reached different conclusions about the sensitivity
of fecal occult blood testing because they used different definitions of sensitivity (22,23).
of fecal occult blood testing because they used different definitions of sensitivity (22,23).
The apparent sensitivity of a test may be influenced by serendipity (24,25). A screen-positive participant in a fecal occult blood screening program might be found to have a small adenoma on colonoscopy. Because the purpose of the screening program is to detect early disease, and because some adenomas progress to cancer, the program would take credit for an adenoma discovered following colonoscopy for a positive fecal occult blood test. The likelihood, however, is that the adenoma was not really responsible for the bleeding that led to the colonoscopy, and the positive fecal occult blood test result was really a false positive. Serendipitous findings of this type inflate the apparent sensitivity of the test.
A sensitive test may also uncover lesions that would never progress to advanced cancer. These findings have been termed pseudodisease (6). The problem with pseudodisease is that it leads to the expense and possible complications associated with treatment. For example, the natural history of Barrett’s esophagus is poorly understood. Barrett’s esophagus patients with dysplasia may be subjected to ablative therapy using heat probes, lasers, or photodynamic therapy. Ablative therapy may result in strictures or perforation. Perhaps such therapy is justified for dysplasia, but it is difficult to make decisions about the value of ablative therapy because our understanding of the natural history of Barrett’s esophagus is incomplete.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree