Gastrointestinal Cancer: Pathology and Molecular Pathology
Alessandro Lugli
Jeremy R. Jass
Introduction
“Frequently consider the connection of all things in the universe and their relation to one another” wrote the Roman Emperor Marcus Aurelius (121–180 AD) in his Meditations (1). This quotation can be applied to the current management of human cancer, whereby practitioners from different disciplines work together to ensure the highest quality of treatment for their patients. Because pathology is the branch of medicine that studies the mechanisms of cell, tissue, and organ injury that underlie a disease process, the pathological evaluation of tumor tissue is an essential component of the management of patients with gastrointestinal cancer, from initial diagnosis through the various stages of treatment.
The pathologist’s tumor diagnosis is generally based on classification schemes that encompass reproducible clinical, morphologic, and molecular criteria. These features provide the diagnostic signatures of human cancers. Tumor classifications are by their nature artificial because biological processes occur as continua. They are also provisional and susceptible to change in the face of new information and evidence. Nevertheless, highly successful and widely accepted classifications of human tumors have been published in a series by the World Health Organization (WHO) and in a systemic series of fascicles by the U.S. Armed Forces Institute of Pathology. The typing of neoplasia is based on histogenesis or comparison with a normal tissue counterpart.
The diagnosis of cancer may be suspected on the basis of a patient’s symptoms, the findings on clinical examination, and imaging modalities such as computed tomography, ultrasound scanning, magnetic resonance imaging, and barium studies. However, a tissue diagnosis based on the morphologic evaluation of a tissue section stained with hematoxylin and eosin (H&E) is the gold standard for the diagnosis of malignancy. The first task of the pathologist is to differentiate a reactive process from neoplasia. Second, benign neoplasia must be distinguished from a malignant process that is characterized by the invasion and destruction of local tissues as well as the potential for metastasis. Sometimes, the differentiation between a primary tumor and a metastasis is not possible with an H&E stain. Ancillary studies using special stains, immunohistochemistry, or molecular analysis may assist in this fundamental distinction, and may contribute clinically useful information on the type, grade, and potential behavior of a tumor.
A uniform and standardized system for classifying the extent of malignancy (staging) is essential to compare therapeutic intervention and estimate outcome. The American Joint Committee on Cancer (AJCC), in cooperation with the TNM (tumor, node, metastasis) Committee of the International Union Against Cancer (UICC), has incorporated factors in relation to tumor spread into a comprehensive TNM staging system (2,3). For most organs, the size of the tumor at its primary site and/or the involvement of local structures describe the tumor topography (T). The presence and extent of regional lymph node (N) involvement and whether there is evidence of distant metastasis (M) indicate the tumor spread. The categories for T, N, and M are condensed into stages 0 to IV, which are the most important determinants of prognosis (2). For example, the prognosis of advanced gastric cancer (spread beyond submucosa or at least T2) is poor, and even after curative resection, the 5-year survival rate ranges from 26% to 35% (4).
The emerging knowledge on the molecular pathogenesis of gastrointestinal neoplasias has lead to the exploration of molecular alterations that could be used to improve diagnosis and management. For example, KIT alterations distinguish gastrointestinal stromal tumor (GIST) from other benign or malignant types of stromal tumors, whereas the immunohistochemical detection of proteins encoded by the mismatch repair genes, notably MSH2, MLH1, and MSH6, have been linked with hereditary forms of colorectal cancer.
Clinical symptoms are often forgotten tumor-related prognostic factors. The presence of tumor-related symptoms such as weight loss, obstruction, and perforation fever are important prognostic factors in most patients with cancer (5,6,7,8). Napoleon Bonaparte (1769–1821), who was afflicted with an advanced gastric cancer, presented with weight loss, fever, and night sweats (9,10).
This chapter introduces the reader to the general principles of gross pathology, histopathology, and molecular concepts that are essential for the diagnosis, classification, and prognostication of gastrointestinal neoplasms.
Pathology of Gastrointestinal Malignancies
Diagnosis of Malignant Gastrointestinal Tumors
The diagnosis of cancer includes the examination of cytologic and tissue specimens for features that differentiate a reactive process from benign or malignant neoplasia. Aspiration or core needle biopsy, biopsy, cell brushing, or tumor resection are procedures for obtaining samples of cells and tissues. The diagnosis
of tumors as benign or malignant is primarly based on cytologic and histologic criteria. Neverthless, immunocytochemistry (performed on cytologic preparations) and immunohistochemistry are complementary and useful tools, not only in the diagnosis of cancer but also in the differentiation between a primary malignant tumor and a metastatic process.
of tumors as benign or malignant is primarly based on cytologic and histologic criteria. Neverthless, immunocytochemistry (performed on cytologic preparations) and immunohistochemistry are complementary and useful tools, not only in the diagnosis of cancer but also in the differentiation between a primary malignant tumor and a metastatic process.
Cytologic Assessment of Gastrointestinal Malignancy
The use of cytology for diagnosis of gastrointestinal tumors varies according to institutional practice and available expertise. The location and type of malignancy may also determine the relative appropriateness of cytologic examination. In general, cytologic specimens may be acquired via brushing or lavage from luminal tumors approached endoscopically, via aspiration of fluid from cystic tumors (e.g., in the pancreas or liver), or via fine-needle biopsy. Tumors of the tubal gut (except the jejunum and most of the ileum), papilla of Vater, and pancreaticobiliary ducts may be approached endoscopically from the lumen and brushed to collect cells from a broad surface area. This technique may be especially useful when luminal stricturing limits access to the tumor for forceps biopsy. Acquisition of cytologic specimens from pancreatic and hepatic tumors often requires the use of ultrasonic or other radiologic guidance techniques. Overall, cytologic approaches may allow for preoperative diagnosis of tumors that are otherwise inaccessible to standard biopsy or that pose particular risks of biopsy-associated complications.
Cytologic diagnosis is limited almost exclusively to identification of tumor and determination of tumor type. Preservation of tissue architecture is limited in cytologic specimens; therefore, assessment for invasion is not possible. In addition to aiding the initial diagnosis, cytologic approaches may be useful in staging of gastrointestinal malignancy. For example, in the TNM staging system for colon cancer of the AJCC and UICC, tumor cells in peritoneal fluid are classified as distant metastasis by convention (2). Thus, positive peritoneal cytology would establish a tumor as stage IV.
Histologic Assessment of Gastrointestinal Malignancy
Masses discovered by clinical examination, imaging, or endoscopic studies that are suspicious for malignancy typically require biopsy confirmation before treatment is initiated. The role of the biopsy is twofold: (a) to exclude the presence of benign lesions that may mimic malignancy clinically, and (b) if malignant tumor is present, to determine the histologic type. The majority of gastrointestinal tumors are carcinomas, and outside the esophagus and anus, they are mainly adenocarcinomas. A large number of other malignant tumors, however, may clinically resemble gastrointestinal carcinomas. These include lymphomas, neuroendocrine tumors, mesenchymal tumors (e.g., GIST), metastatic tumors that exhibit tropism for the gastrointestinal tract (e.g., melanomas), and malignancies of adjacent organs that directly invade the gastrointestinal tract (e.g., cancers of the ovary, endometrium, urinary bladder, or prostate). Benign lesions that may mimic gastrointestinal carcinomas include adenomas, hamartomas, benign ulceration (usually due to ischemia or inflammatory processes, such as Helicobacter pylori infection in the stomach or cytomegalovirus infection in the colon), endometriosis, inflammatory conditions such as inflammatory bowel disease (Crohn’s disease or ulcerative colitis), solitary rectal ulcer syndrome, and diverticular disease with mural stricturing.
Multiple biopsy specimens taken from the edges and base of an ulcerating lesion or from the surface of a polypoid mass typically reveal the correct diagnosis. When an obstructing mass is present, however, passage of an endoscope to obtain diagnostic tissue may be difficult; thus, brush cytology may be useful to confirm the diagnosis in this situation. Even when direct access to the tumor is possible, biopsy specimens may fail to yield a definitive diagnosis if the lesion is extensively ulcerated or otherwise necrotic and viable tumor tissue is not obtained on sampling. The diagnostic yield is improved when multiple biopsy samples are taken, and for ulcerated lesions, when samples are obtained from the ulcer edge at all four quadrants and from the ulcer center. For ulcerated carcinomas, biopsy specimens obtained from the periphery of the ulcer are likely to contain viable tumor. In contrast, for ulcerated lymphomas and sarcomas, biopsy samples obtained from the center of the lesion are likely to contain diagnostic tissue.
Even when incisional biopsies (i.e., diagnostic needle or endoscopic forceps biopsies) are successful, the type and amount of information that can be derived from the specimen is limited. Pathological confirmation of the presence of malignancy and its histologic type may be unequivocally established with an adequate biopsy, but the tumor grade and presence of invasion (of carcinomas) may be difficult or even impossible to determine. Even for carcinomas in which stromal invasion is identified with certainty, the deepest extent of invasion cannot be determined from incisional biopsy material. Furthermore, luminal biopsies from the tubal gut or the papilla of Vater, extrahepatic bile ducts, and pancreatic ducts are typically limited in depth to the superficial mucosa or deep lamina propria, respectively. Thus, tumors with primarily submucosal or mural growth, such as lymphomas, neuroendocrine tumors, and sarcomas, may be missed on luminal biopsy unless a single biopsy site is sampled repeatedly to obtain deeper tissue.
Immunohistochemical Assessment of Gastrointestinal Malignancy
Morphologic assessment may not be diagnostic in the case of poorly differentiated tumors. Additional techniques, including special histochemical stains, immunohistochemical stains, or electron microscopy, may be required to detect specific features of cell lineage or differentiation. Because electron microscopy is labor intensive, several days may be required to obtain results. It also requires tissue fixation in glutaraldehyde rather than 10% formalin for optimal preservation of ultrastructural features. For these reasons, electron microscopy is rarely adopted as the technique of choice for additional studies on diagnostic biopsy specimens. In contrast, special histochemical stains can be performed within hours on fixed tissue. For example, neutral and acidic mucins (adenocarcinoma), glycoproteins (adenocarcinoma or hepatocellular carcinoma), neurosecretory granules (neuroendocrine tumors), melanin (primary or metastatic melanoma), and other tumor cell products or associated proteins can be identified by special stains.
Immunohistochemical stains are now in common use and constitute the most powerful tools in the diagnostic armamentarium of the surgical pathologist. Compared with electron microscopy and special stains, immunohistochemistry is both more sensitive and more specific for revealing differentiation markers. Because the standard procedure in most institutions is to fix diagnostic biopsy specimens immediately in 10% formalin, antibodies that can be used on paraffin-embedded, formalin-fixed tissue are the most useful and widely used. Some antigens, however, are altered by fixation or heat (required for paraffin embedding), and fresh tissue may be required for their immunolocalization. Gastrointestinal lymphomas, for example, may require fresh tissue for immunolabeling of light chains to demonstrate clonality. There is increasing availability, however, of commercially available monoclonal antibodies that give excellent results with formalin-fixed tissues. It is absolutely essential that immunohistochemical analysis of a tumor is used in the context of a carefully selected differential diagnosis based on clinical data and morphologic features of the tumor.
Table 2.1 Immunohistochemical Stains used in the Diagnosis and Differential Diagnosis of Gastrointestinal Tumors | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||
One of the most challenging problems for a surgical pathologist is the diagnosis of a poorly differentiated tumor when no primary site is clinically evident. Fortunately, malignant cells, even when poorly differentiated, continue to express antigens that characterize the cell or tissue of origin. Lineage-dependent expression of proteins is therefore maintained in most poorly differentiated cancers. Immunohistochemical analysis with appropriate antibodies can usefully differentiate, for example, epithelial, mesenchymal tumors, neuroendocrine tumors, lymphomas, and melanomas. Pancytokeratin markers such as LU5 or CK22 can distinguish carcinomas (positive) from other poorly differentiated tumors (negative). The latter group includes the differential diagnosis of neuroendocrine tumors (positive for synaptophysin-, chromogranin-, and neuron-specific enolase), melanomas (positive for HMB45, S-100, and MelanA), and lymphomas (positive for CD45). Sarcomas of the gastrointestinal tract are recognized with antibodies to desmin (positive in smooth and skeletal muscle tissue), actin (smooth muscle tissue), and vimentin. GIST are typically positive for CD117 (KIT) and CD34.
In addition, cytokeratins (CKs) have a diverse and lineage-specific expression pattern that has been shown to identify the site of origin of many epthelial tumors. The CKs include at least 20 different polypeptide chains, and two-dimensional gel electrophoresis studies have shown that these are distributed in a relatively tissue-specific manner. The CK profile of the epithelial tissue of origin is retained by the tumor. For example, more than 95% of adenocarcinomas of the colon are CK20 positive but CK7 negative. This may assist with the distinction from ovarian cancer, which is usually CK7 positive and CK20 negative. CDX2 is a homeobox domain-containing transcription factor important in the development and differentiation of the intestine. It serves as a sensitive and specific marker for colorectal adenocarcinoma and is also helpful in distinguishing adenocarcinomas of the papilla from those arising in the pancreas and biliary tree (11). Metastatic prostate cancer can be recognized by positivity for prostatic-specific antigen, and metastases of breast cancers are often positive for estrogen and/or progesteron receptors. In addition, but more rarely, metastasis from lung cancers (TTF positive), renal cancers (RCC positive), and mesothelioma (calretinin positive) have to be considered in the differential diagnosis. Some of the more commonly used antibodies in gastrointestinal tumor diagnosis are summarized in Table 2.1.
Unfortunately, the initial impression of high specificity for a particular marker of differentiation is frequently tempered by the test of time. In light of the inevitable limitation of a particular antibody with respect to its final utility as a diagnostic marker for a specific tumor type, there is a clear need for early and comprehensive analysis of novel diagnostic markers in both normal and neoplastic tissues. If traditional methods are used, such an evaluation would require the immunohistochemical interpretation of thousands of samples, a task that could not be completed by one laboratory in a reasonable time frame. Tissue microarray (TMA) technology is ideal for the standardized and high-throughput molecular analysis of new monoclonal antibodies (12,13,14,15). To analyze the specificity of an antibody in different normal and neoplastic tissue types, a set of TMAs
containing either different tumor types or numerous examples of the same tumor type, as well as normal tissue samples, are prepared (16,17) (Figure 2.1). Beginning with archival specimens of formalin-fixed, paraffin-embedded tissue blocks, tissue cylinders with a diameter of 0.6 mm are punched from representative areas of each “donor” tissue block. These are then incorporated into a recipient paraffin block (3 × 2.5 cm) using, for example, a semiautomated tissue arrayer. The immunohistochemical results obtained by this method can be correlated with clinicopathological patient data to establish whether the marker is prognostic or predictive (18). In addition, the diagnostic utility of an antibody can be analyzed and characterized with respect to its immunohistochemical patterns. In a recent study, expression of hepatocyte paraffin 1 was found not only in hepatocellular carcinoma but also in gastric, small intestine, gallbladder, and adrenal gland carcinoma (19). Therefore, other markers would be required to distinguish these tumors: AFP, CD10, and p-CEA in hepatocellular carcinoma; CK7, CK13, and BerEp4 in gastric cancer; CK7, CK17, and BerEp4 in gallbladder carcinoma; CK13 and CK20 in small intestine carcinoma and vimentin; and MelanA and synaptophysin in adrenal gland carcinoma (19).
containing either different tumor types or numerous examples of the same tumor type, as well as normal tissue samples, are prepared (16,17) (Figure 2.1). Beginning with archival specimens of formalin-fixed, paraffin-embedded tissue blocks, tissue cylinders with a diameter of 0.6 mm are punched from representative areas of each “donor” tissue block. These are then incorporated into a recipient paraffin block (3 × 2.5 cm) using, for example, a semiautomated tissue arrayer. The immunohistochemical results obtained by this method can be correlated with clinicopathological patient data to establish whether the marker is prognostic or predictive (18). In addition, the diagnostic utility of an antibody can be analyzed and characterized with respect to its immunohistochemical patterns. In a recent study, expression of hepatocyte paraffin 1 was found not only in hepatocellular carcinoma but also in gastric, small intestine, gallbladder, and adrenal gland carcinoma (19). Therefore, other markers would be required to distinguish these tumors: AFP, CD10, and p-CEA in hepatocellular carcinoma; CK7, CK13, and BerEp4 in gastric cancer; CK7, CK17, and BerEp4 in gallbladder carcinoma; CK13 and CK20 in small intestine carcinoma and vimentin; and MelanA and synaptophysin in adrenal gland carcinoma (19).
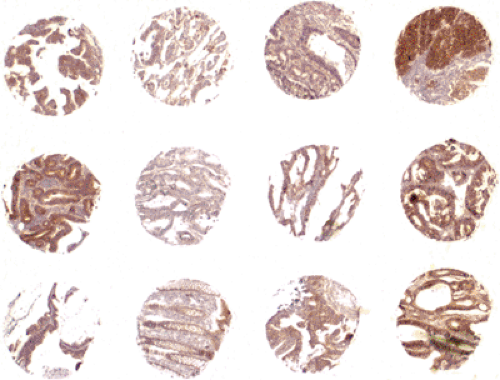 FIGURE 2.1. Tissue microarray showing differing intensity of epidermal growth factor receptor immunohistochemistry in multiple colorectal cancer punches (2×). Source: From the Archives of the Institute of Pathology, University Hospital Basel, Institute of Clinical Pathology, Basel and Institute of Pathology, Stadtspital Triemli, Zürich, Switzerland (See also color Figure 2.1). |
Apart from its application to immunohistochemistry, microarray technology allows the expression of thousands of genes within sets of tumors to be analyzed simultaneously. The first example of this revolution introducing a molecular as opposed to morphologic classification of tumors is seen in the analysis of lymphomas by Alizadeh et al. (20). Particular patterns of gene expression clusters may be correlated with morphologic, prognostic, or predictive outcomes to provide novel types of classification. However, it is likely that morphologic classifications will remain the gold standard for some time.
Classification of Malignant Gastrointestinal Tumors
The morphologic classification of tumors has evolved with the increased understanding of tumorigenesis. The classification of malignant gastrointestinal neoplasias has traditionally occurred at two levels: Macroscopic and microscopic.
Macroscopic Classification of Malignant Gastrointestinal Tumors
Despite the modern dominance of histologic features in achieving a final diagnosis, the macroscopic description of tumors on the basis of configuration, size, and anatomic site remains the first step in pathological examination. Classically, malignant tumors are solid, nonencapsulated, and characterized by large size, irregular and infiltrative borders, and presence of necrotic areas. An early example of a macroscopic classification is that of Borrmann. This subdivides advanced gastric cancer into four types: type I (polypoid), type II (fungating), type III (ulcerated), and type IV (infiltrative) (21). Types I and III occur commonly in the body, mainly the greater curvature, and account for about 25% of gastric carcinomas, whereas type II is frequently found in the antrum, along the lesser curvature, and represents approximately 35% of cases (22). Type IV is also known as linitis plastica.
Macroscopic features have been used to distinguish a benign gastric ulcer from an ulcerated gastric carcinoma (type III). Gastric ulcers are usually small, well-circumscribed, punched-out lesions with a clean base and smooth, oedematosus margins. In contrast, ulcerated carcinomas have irregular borders, which are firm, fixed, and often raised, and the ulcer base is typically necrotic and hemorrhagic (23) (Figure 2.2). As stated by Ming and Goldman, careful inspection usually allows the observer to distinguish a malignant from a benign ulcer (24). A more contemporary contribution is the endoscopic classification of early gastric cancer (EGC) proposed by the Japan Gastroenterological Endoscopic Society (25). This system was developed in parallel with improving technology and increasing use of upper endoscopy, first in Japan and then worldwide (26,27,28,29). EGC is defined as invasive adenocarcinoma confined to the mucosa or submucosa with or without lymph node metastasis. EGC is subdivided into three types: Protruded (type I), superficial (type II), and excavated (type III). Type II is further subdivided into an elevated type IIa, flat type IIb, and depressed type IIc. The importance of recognizing an EGC lies in the fact that gastric surgery is then indicated and is usually curative (30).
The macroscopic classification of colorectal cancer (CRC) is similar to gastric cancer (31). (a) Exophytic, polypoid tumors typically occur in the cecum, rarely result in obstruction,
and often achieve a large size before clinical presentation. (b) Ulcerating and infiltrative tumors are characterized by raised, irregular edges and a central ulcer. (c) Annular and constricting tumors often result in desmoplasia, which results in a firm consistency, proximal dilatation due to a functional obstruction and a characteristic double-contrast “apple-core” lesion. (d) Diffuse tumors infiltrate the entire bowel wall and are analogous to linitis plastica of the stomach.
and often achieve a large size before clinical presentation. (b) Ulcerating and infiltrative tumors are characterized by raised, irregular edges and a central ulcer. (c) Annular and constricting tumors often result in desmoplasia, which results in a firm consistency, proximal dilatation due to a functional obstruction and a characteristic double-contrast “apple-core” lesion. (d) Diffuse tumors infiltrate the entire bowel wall and are analogous to linitis plastica of the stomach.
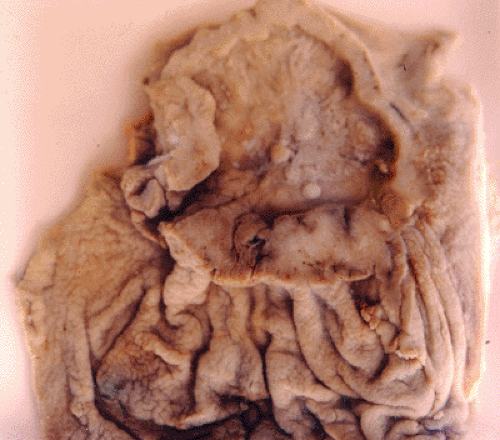 FIGURE 2.2. Gastric cancer, ulcerated type (Borrmann III). The ulcer base is necrotic, and the tumor borders are irregular and have been trimmed to show pale cancer tissue (See also color Figure 2.2). |
Although the gross appearance is variable, the cut section of all macroscopic types is normally homogenous, comprising firm pale tissue admixed with necrotic areas. There is no evidence that gross configuration is a prognostic indicator independent of the underlying histologic subtype (32). In contrast, anatomic site has been linked to prognosis. Tumors located in the cecum, ascending hepatic flexure, and transverse colon may be termed right sided, whereas tumors in the splenic flexure, descending, or sigmoid colon are grouped as left sided. Right-sided colon carcinomas have a better prognosis than left-sided ones (33). The main reason is probably related to the molecular pathogenesis rather than site per se because CRCs with microsatellite instability (MSI) tend to occur in the right colon.
Overall, the diagnostic, predictive, and prognostic importance of the macroscopic features of gastrointestinal cancers is limited, and histologic examination is mandatory in every case. Nevertheless, although the final classification is based on histologic characteristics, the absolute dependence of staging on meticulous dissection of surgical specimens should not be overlooked.
Histologic Classification of Malignant Gastrointestinal Tumors
For consistency and uniformity in pathological reporting of gastrointesinal malignancies, the use of internationally accepted terminology and diagnostic criteria is encouraged by the College of American Pathologists (CAP) and other professional bodies. On this basis, the standardized tumor classification for intestinal, hepatic, biliary, and pancreatic neoplasms established by the WHO is recommended (34). Tumors are classified on the basis of the tissues generated by the cancer. This approach is a histogenetic classification that may be addressed at four levels: (a) tissue of origin, (b) histologic subtype, (c) growth pattern, and (d) tumor grade.
Tissue of Origin
Two major classes of tissue are distinguished: Epithelium and connective tissue. Malignant tumors that arise from the epithelial cells are carcinomas, whereas those from the supporting tissues derived from the embryonic mesodermal layer are sarcomas. Cancers arising in connective tissue, fat, smooth muscle, skeletal muscle, vessels, cartilagenous tissue, and bone tissue are termed fibrosarcomas, liposarcomas, leiomyosarcomas, rhabdomyosarcomas, angiosarcomas, chondrosarcomas, and osteosarcomas, respectively. Haematopoietic and lymphoid cells give rise to leukemias and lymphomas. Most gastrointestinal cancers are carcinomas, followed by lymphomas and sarcomas.
Histologic Subtype
Carcinomas with a glandular growth pattern are adenocarcinomas, whereas those with squamous differentiation are squamous cell carcinomas. Adenocarcinomas comprise various histologic subtypes that may be illustrated with reference to the WHO classification of colorectal tumors. The usual type is characterized by medium to large glands, with moderate variability in gland size and configuration, and only moderate amounts of stroma. Mucinous adenocarcinoma is defined by the presence of abundant secretory mucin accounting for ≥50% of the tumor. The term “adenocarcinoma with mucinous differentiation” applies to tumors that show a significant mucinous component (>10% but <50%). Signet ring cell adenocarcinoma is composed of at least 50% signet ring cells. Histologically, tumor cells show a characteristic mucin vacuole that pushes the nucleus to the periphery of the cytoplasm. Although there is usually good concordance between types of normal epithelium and types of carcinoma, squamous cell carcinoma, adenosquamous carcinoma, and carcinoma with squamous metaplasia may occasionally occcur in the colon.
Growth Pattern
Malignant tumors within a given category display characteristic microscopic architectural patterns that can serve as clues to their histogenesis. The architectural pattern is often documented in the histologic pathological examination of tumor biopsies or tumor resection specimens. A tubular pattern refers to distended or anastomosing branching tubules of various sizes, whereas a papillary pattern shows epithelial projections with central fibrovascular cores. These two variants are encountered in the WHO classification of gastric adenocarcinoma. Tumors that grow in either solid or trabecular patterns include medullary carcinoma of the colon, neuroendocrine tumors, and hepatocellular carcinoma. Cystic growth is distinctive but relatively uncommon in gastrointestinal tumors, occurring in some mucinous carcinomas and endothelial (i.e., lymphangiomatous or hemangiomatous) tumors. In the pancreas, epithelial tumors with a cystic configuration occur relatively frequently. Solid tumors of any histologic type, however, including stromal tumors, lymphomas, and carcinomas, may undergo secondary cystification due to central necrosis, an occurence that may lead to misdiagnosis as a primary cystic neoplasm on imaging studies. Recently, colorectal carcinomas with a serrated glandular architecture have been linked with serrated polyp precursor lesions (35).
In general, the tumor growth pattern has little independent prognostic value in gastrointestinal malignancies (36,37). However, histologic subtype, growth pattern, and gross tumor configuration may be grouped together to give a useful global classification. With the Lauren classification, for example, gastric cancer is subdivided into three types: Intestinal, diffuse, and indeterminate/unclassified (38). The macroscopic appearance described as linitis plastica equates with the diffuse type of carcinoma that often shows signet ring cells and has a highly unfavorable prognosis (39). In CRC, tumor budding (single cells or clusters of up to four cells at the invasive tumor margin) has be shown to be associated with a diffuse pattern of
infiltration and to confer a worse prognosis (40,41,42,43,44,45). These examples show that architectural patterns and growth characteristics may be merged to give complex but useful histologic classifications.
infiltration and to confer a worse prognosis (40,41,42,43,44,45). These examples show that architectural patterns and growth characteristics may be merged to give complex but useful histologic classifications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








