Gastrointestinal Cancer: Nutritional Support
Joel B. Mason
Protein-calorie malnutrition, typically referred to simply as malnutrition, is a common but not invariable feature of cancer, the most frequent manifestation of which is weight loss. A large, multicenter survey of more than 3,000 patients awaiting the initiation of chemotherapy, for instance, observed weight losses exceeding 4% in one-third of the patients and, in one-half of these, the magnitude of loss exceeded 10% (1). Weight losses exceeding 10% are of particular significance because there is considerable evidence that weight loss of this magnitude in the setting of illness leads to significant increases in morbidity and mortality (2).
The likelihood that a particular individual will sustain substantial weight loss is related to many factors: the type of cancer is important, as is the presence of metastases, physical impedance of normal food intake by the cancer, and the presence of intervening emotional issues such as depression. In the aforementioned survey (1), only 4% to 7% of individuals with leukemias, sarcomas, and breast cancer experienced more than 10% weight loss, whereas individuals with gastrointestinal tract cancers had a much higher likelihood of this degree of weight loss: 14% of patients with colon cancer and 25% to 40% of individuals with pancreatic and gastric cancers showed such a loss. Not surprisingly, carcinoma of the oropharynx, which frequently interferes with the processes of chewing and swallowing, results in more than 10% weight loss in more than 40% of patients (3).
Nevertheless, arriving at precise figures for the prevalence of significant protein-calorie malnutrition among cancer patients is a frustrating task because it depends on the particular assessment tool that is used to define malnutrition. For instance, another survey that used creatinine-height index (a measure of muscle mass) as the criterion for protein-calorie malnutrition observed that 90% of hospitalized cancer patients are significantly malnourished (4). Ultimately, it is perhaps more constructive merely to be aware that malnutrition of a degree that is associated with worse clinical outcomes is a common phenomenon among cancer patients, that it is particularly common in cancers of the gastrointestinal tract, and that physicians need to be cognizant of this reality in their assessment and management of the patient.
Unequivocal proof that malnutrition independently contributes to the morbidity, mortality, and diminished quality of life among cancer patients does not exist, but this contention is almost certainly true. A plethora of case-control and prospective cohort studies in cancer patients indicate that a substantial degree of malnutrition diminishes tolerance of and responsiveness to chemotherapy (1,5) and radiotherapy (6), increases perioperative morbidity (7), worsens the quality of life (5), and diminishes the likelihood of survival (1,5,8,9,10). Most important, clinicians should realize that appropriate and prompt attention to meeting the nutritional needs of malnourished patients improves the clinical outcome of many types of ill patients, as has been shown repeatedly in prospective, randomized trials (reviewed in 11). Improvements in clinical outcome as a result of aggressive nutritional support have been more difficult to demonstrate in the cancer patient, probably because other factors have such a major impact on the clinical course. Nevertheless, aggressive nutritional support has genuine benefits to offer the cancer patient in selected circumstances. A detailed discussion of these circumstances appears later in this chapter.
Malnutrition in the Cancer Patient: Mechanisms
The development of malnutrition in the cancer patient is usually multifactorial, and an appreciation of such multiplicity in etiology is necessary if one is to design an effective approach to treatment. Table 8.1 outlines factors that are often observed to contribute to this problem.
Body Compartment Perspective
The type of tissue that is lost when an individual loses weight is critical in determining the pathological ramifications of the weight loss. The vast majority of the metabolic machinery that maintains normal homeostasis resides in the lean body mass, and maintenance of this body compartment is most critical for health. The lean body mass can be further subdivided into skeletal muscle mass; visceral lean mass (which includes the major organs); and extracellular lean mass, such as the interstitial fluid, blood serum, and the mineral matrix of the skeleton (Fig. 8.1). These are useful distinctions because the body draws on each of these compartments in a different fashion in the setting of weight loss. In the cancer patient, there is usually a disproportionately large contraction of the skeletal muscle mass and fat mass with relative sparing of the visceral mass, and in this respect, the weight loss is similar to that seen in many acute illnesses of a nonmalignant nature (12). For example, in one study of cancer patients who had lost, on average, one fourth of their preillness weight, fat mass and skeletal muscle mass each decreased by 75% to 80% of control values, whereas visceral lean mass was spared and did not decrease to a significant degree (13). By comparison, the weight loss in simple starvation is less detrimental because the body preferentially uses adipose tissue for energy needs; therefore, the percentage loss in skeletal muscle mass is considerably less than the proportional loss of fat mass. For example, in healthy volunteers fed a calorically
inadequate diet for 3 months, approximately one fourth of the initial body weight was lost; this was accompanied by a 70% decline in fat mass but only a 24% drop in lean mass (14). Table 8.2 summarizes the relative losses in these body compartments observed in simple starvation versus those observed in the wasting associated with cancer.
inadequate diet for 3 months, approximately one fourth of the initial body weight was lost; this was accompanied by a 70% decline in fat mass but only a 24% drop in lean mass (14). Table 8.2 summarizes the relative losses in these body compartments observed in simple starvation versus those observed in the wasting associated with cancer.
Table 8.1 Factors that contribute to the development of protein-calorie malnutrition in the cancer patient | |
|---|---|
|
The weight loss and muscle dissolution (the combination of which is now referred to as wasting [15]) that one sees in cancer can be perceived as a physiological adaptation to stress; that is, the body sacrifices large portions of the muscle mass to spare more immediately critical functions in the visceral mass. However, there are clear limitations to this adaptive response. First, contraction of the skeletal muscle mass leads to muscle weakness, decreased work tolerance, and measurable decreases in functional status (16). Second, sparing of the visceral mass is only relative, and sustained weight loss will eventually lead to contractions of this compartment as well.
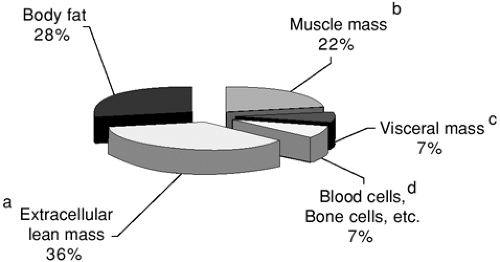 FIGURE 8.1. Typical body composition analysis by weight in a healthy adult. Segments a through d collectively represent lean body mass; segments b through d alone represent body cell mass. |
Table 8.2 Comparison of body compartment losses in starvation and cancer wasting | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Mechanisms of Wasting in Cancer
Anorexia
Anorexia commonly contributes to wasting in cancer. Particularly with cancers of the gastrointestinal tract, the act of eating may incite a variety of adverse symptoms (including pain, vomiting, and diarrhea); anorexia may therefore evolve as a learned means of avoiding such symptoms. The tumor mass may also preclude adequate ingestion of food, a factor that is particularly prevalent in cancers of the oropharynx, esophagus, and stomach. In addition, therapeutic modalities involving drugs, radiation, or surgery can directly induce anorexia or deter the patient from eating to avoid the gastrointestinal side effects of therapy. A prime example of this is chemotherapy-induced mucositis. The emotional adjustment associated with dealing with a major cancer continues to be a common precipitant of depression and anxiety (17), and these emotional states can also be important in producing a state of anorexia.
Nevertheless, anorexia is commonly present, even in the absence of any of the previously mentioned factors, and may even be the presenting symptom of the cancer. Anorexia in such a setting is believed to be due largely to the effects of tumor-associated cytokines that are known to induce anorexia (Table 8.3) and that are believed to chiefly originate from host cells that are descendants of the white blood cell lineage (macrophages and lymphocytes), which are responding to the presence of the neoplasm. A highly reproducible and remarkable degree of anorexia is observed with administration of tumor necrosis factor-α (TNF-α) (18), interleukin (IL)-1 (19), and interferon-γ (20).
Alterations in Metabolism
A wide spectrum of alterations in protein, lipid, and carbohydrate metabolism are commonly observed in patients bearing cancers (Table 8.3). In concert with the other factors outlined in Table 8.1, these factors contribute to the development of malnutrition. Although much of the work pertaining to mechanisms has been performed in models other than the intact human (human and nonhuman cell culture and animal models), studies in humans have been in general accord with the nonhuman models.
Effects on Protein Metabolism and Lean Body Mass
The skeletal muscle is where most of the initial contraction of lean body mass occurs in the wasting associated with cancer (13). The extent to which this compartment is diminished inversely correlates with the likelihood of survival, underscoring the import of this phenomenon (21). The contraction of skeletal muscle mass appears to be due to both a reduction in protein synthesis and an increase in protein degradation (22). Total body protein turnover is usually observed to be increased in this setting, and this increase is often present even before clinically evident wasting has occurred (23,24).
Table 8.3 Major cytokines believed to be involved in cancer anorexia and wasting | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TNF-α and IL-6 appear to play major roles in mediating the dissolution of skeletal muscle in the wasting associated with cancer, and IL-1 and interferon-γ probably also play significant roles. Exogenous administration of TNF-α and IL-6 has these effects on skeletal muscle or its components (25,26), an effect that is overcome by specific antibodies directed against TNF-α (27). TNF-α and IL-6 are likely not the direct effectors of the response; rather, they probably act by stimulating the secretion or expression of more “downstream” mediators. In some instances, the neoplasm itself appears to be the source of the factor that mediates cachexia; proteolysis-inducing factor (PIF), a complex glycoprotein, promptly induces proteolysis in isolated muscle preparations and a reduction in lean mass in intact animals (28). It has been found in the urine of cancer patients with weight loss but not in that of those without loss of weight (29). It is not found in the urine of patients who have lost weight due to nonneoplastic illnesses. Thus, this factor appears to be highly specific for muscle wasting in cancer. Existing evidence suggests that PIF predominantly originates in the cells of the neoplasm, and among patients with gastrointestinal cancers, PIF expression in the tumor correlates with weight loss (30). Furthermore, in human prostate cancer, messenger RNA for PIF is localized solely to the epithelial cells of the cancer and not in the stromal cells or normal prostate tissue (31). Nevertheless, observations regarding PIF have been somewhat difficult to reproduce; thus, its role in cancer wasting remains a matter of debate.
Effects on Lipid Metabolism and Adipose Tissue
In the wasting associated with cancer, the body continues to use adipose tissue as a major source of energy (although not as predominantly as in simple starvation), and therefore, a decrease in fat mass is usually seen. The net efflux of glycerol and fatty acids from the adipose tissue associated with use of this tissue appears to be due to at least three factors: (i) an increased rate of lipolysis in adipose tissue, which is apparently mediated through TNF-α, leukemia inhibitory factor, and lipid-mobilizing factor (32,33,34); (ii) a decrease in de novo lipogenesis in the adipose tissue, mediated largely by TNF-α and IL-1 (35); and (iii) diminished activity of lipoprotein lipase, which is necessary for the uptake of fatty acids from circulating lipoproteins and which appears to be mediated by TNF-α, IL-6, interferon-γ, and leukemia inhibitory factor (36,37). The decrease in lipoprotein lipase, in particular, explains why cancer patients have a diminished ability to clear an exogenous lipid load (38) and often have elevated plasma glycerol and triglyceride levels (39). Hypertriglyceridemia in cancer patients may also be related to increased rates of hepatic lipogenesis because several of the cytokines implicated in cancer wasting, including TNF-α, IL-1, and interferon-α, each stimulate hepatic lipogenesis (40). Like proteolysis-inducing factor, lipid-mobilizing factor is produced by cells of the neoplasm, although it differs in that its expression can also be induced in
normal adipose tissue of animals on implantation of a cachexia-inducing tumor (41). Table 8.3 lists the mediators of these alterations in fat metabolism in the cancer patient afflicted with wasting.
normal adipose tissue of animals on implantation of a cachexia-inducing tumor (41). Table 8.3 lists the mediators of these alterations in fat metabolism in the cancer patient afflicted with wasting.
Effects on Carbohydrate and Energy Metabolism
Cancer frequently produces a state in which the host expends more calories per kilogram of lean mass than is normal. This state of hypermetabolism is inherently less energy efficient and, therefore, predisposes to weight loss.
The Cori cycle, whereby lactate produced by the cancer or by peripheral tissues is converted back to glucose in the liver, is an inefficient means of salvaging glucose, consuming six molecules of adenosine triphosphate per cycle. If the cancer or other tissue is producing significant quantities of lactate by anaerobic glycolysis, which yields only two molecules of adenosine triphosphate per molecule of glucose substrate, substantial net loss of energy occurs (a so-called “futile cycle”). Increased activity of the Cori cycle has been reported to exist in individuals with cancer and, more specifically, in those cancer patients with weight loss (42). Nevertheless, the quantitative contribution to cancer wasting made by excessive activity of the Cori cycle is not known.
Other commonly altered aspects of carbohydrate metabolism include increased rates of gluconeogenesis and glucose flux, and the development of impaired insulin secretion as well as a modest degree of insulin insensitivity. The latter produces impaired use of glucose in peripheral tissues and glucose intolerance (43). Similar alterations in glucose metabolism are observed in any condition associated with a systemic inflammatory response and are largely believed to be due to TNF-α (44). These changes contrast considerably with those associated with weight loss unrelated to illness or cancer, in which insulin sensitivity is maintained (43).
Assessment of Nutritional Status: A Brief Introduction
Providing nutritional support in a rational manner requires that the clinician acquire an objective means of systematically categorizing patients into those who are either well nourished or mildly malnourished versus those who have a moderate to severe degree of malnutrition. It is the patients in the latter category who will benefit from an aggressive approach to nutritional support. Patients with moderate to severe malnutrition have demonstrable impairments in many physiologic processes due to the malnutrition and suffer significantly greater morbidity and mortality as a result. Most important, the added morbidity and mortality can be attenuated or eliminated by diligent attention to their nutritional needs (reviewed in 11). Similar salutary benefits of aggressive nutritional support usually cannot be demonstrated in well-nourished or mildly malnourished patients.
A comprehensive assessment of protein-energy status involves taking a history (including a diet history), conducting a physical examination, taking anthropometric measures of nutritional status (e.g., weight, midarm muscle circumference, triceps fat fold), performing biochemical tests such as the measurement of serum albumin or prealbumin, and taking objective measurements of body compartments with tools such as body impedance analysis or dual-photon absorptiometry. The means of performing this type of comprehensive assessment is reviewed elsewhere (45,46,47,48) and is beyond the scope of this chapter. Far simpler algorithms, which are surprisingly accurate, can be used by the clinician if the primary purpose is merely to categorize patients either as well or mildly malnourished versus moderately to severely malnourished.
Perhaps the most straightforward means is to determine the percentage of unintentional weight loss that the patient has suffered as a result of disease. Because disproportionately large degrees of protein catabolism accompany acute inflammatory illnesses and cancer wasting, an unintentional weight loss of 10% or more of premorbid weight due to disease translates into a contraction of 15% to 20% of the critical protein-containing compartment of the body, and beyond this threshold, impaired physiologic functions, as well as increased morbidity and mortality, are observed (2). Clinical trials have repeatedly demonstrated that patients who exceed this threshold benefit from aggressive nutritional support (49,50).
Body weight can, however, be misleading. A common example in gastrointestinal/hepatobiliary malignancies is the patient with cirrhosis and ascites, in whom the weight of the ascites masks the loss in lean body mass. Studies that have used sophisticated means of assessing total body protein have demonstrated that nearly all patients who are categorized into Childs-Pugh class B or class C have lost more than 20% of total body protein; more surprising is the fact that half of patients with Childs-Pugh A classification have also lost this degree of total body protein (51).
Two other commonly used means of assessing protein-calorie status are the creatinine-height index and the prognostic nutritional index (PNI). The creatinine-height index, which is the amount of urinary creatinine excreted in 24 hours corrected for the patient’s height, is an accurate reflection of muscle mass because a constant percentage (approximately 2%) of muscle creatine is converted to creatinine each day. However, incomplete urine collections, excessive meat ingestion, corticosteroid therapy, and abnormal or unstable renal function can each alter apparent or actual creatinine excretion independent of muscle mass. Gender-specific tables exist for normative values, and a patient whose index is 80% or less of the normative value can be considered to have a moderate to severe degree of malnutrition (Table 8.4). The PNI is one of several nutritional indices that represent a weighted regression of nutritional and physiological measures. The PNI has been shown to be a valid predictor of postoperative complications and mortality among inpatient cancer patients who are about to undergo surgery (52). The disadvantage of the PNI is that it requires the measurement of serum albumin and transferrin, triceps skinfold (the accurate measure of which is highly operator dependent), and delayed skin hypersensitivity. Although in reality the PNI is a reflection of both nutritional status and severity of illness, a value greater than 40% suggests moderate to severe malnutrition.
Efficacy of Nutritional Support
Aggressive nutritional support, defined here as the use of whatever means is necessary and practical to meet the nutritional needs of the patient, does not benefit every patient with cancer. Understanding on the part of the clinician as to what can reasonably be expected from a course of aggressive nutritional support is therefore of paramount importance so that an appropriate decision can be made about whether its use is warranted in a particular case.
First, one must recognize that the alterations in metabolism that accompany wasting in cancer make it exceedingly difficult in most patients to correct the existing nutritional deficits. In a patient with an untreated cancer, there is typically little accrual in the critical protein-containing compartment of the body in response to nutritional support; a gain in weight may not occur and, when it does, much of the weight that is
gained is due to water and to an expanded fat mass (53). It is nevertheless true that even in the absence of weight gain, or demonstrable increases in the levels of serum proteins that reflect protein-energy status (e.g., albumin, prealbumin, retinol-binding protein), providing a course of nutritional support to an appropriate patient can improve physiologic functions and clinical outcome. Once a tumor mass has been eliminated or shrunken by treatment, many of these metabolic aberrations disappear (54), and therefore, the expectation that aggressive nutritional support might help replete lean body mass is reasonable.
gained is due to water and to an expanded fat mass (53). It is nevertheless true that even in the absence of weight gain, or demonstrable increases in the levels of serum proteins that reflect protein-energy status (e.g., albumin, prealbumin, retinol-binding protein), providing a course of nutritional support to an appropriate patient can improve physiologic functions and clinical outcome. Once a tumor mass has been eliminated or shrunken by treatment, many of these metabolic aberrations disappear (54), and therefore, the expectation that aggressive nutritional support might help replete lean body mass is reasonable.
Table 8.4 Normative values for creatinine excretion based on height | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The next several subsections review common clinical scenarios in which substantial evidence exists that aggressive nutritional support provides benefit to the patient. Contained in the text accompanying each scenario are qualifications that describe the particular circumstances under which an aggressive approach is indicated.
Cancer Patient Undergoing Major Surgery
The setting in which nutritional support for the cancer patient has been most reproducibly demonstrated as beneficial is that of moderate to severe malnourishment in patients who are scheduled to undergo major surgery. Aggressive nutritional support for 7 or more days before surgery reproducibly reduces perioperative complications, and sometimes mortality, in malnourished patients when examined in a prospective and controlled fashion (49,55,56,57). The Veterans Administration Cooperative Trial (49), a multicenter trial encompassing nearly 500 subjects of whom two-thirds had cancer, demonstrated an important qualification to this benefit. Patients who were categorized as “severely” malnourished and who were randomly assigned to receive preoperative total parenteral nutrition (TPN) experienced a nearly 90% decline in noninfectious perioperative complications, whereas no benefits were observed in mildly malnourished or well-nourished individuals. Consequently, in those trials that are confined to moderately to severely malnourished patients, preoperative nutritional support conveys large benefits. One trial that enrolled 90 patients with gastric or colorectal cancers undergoing surgery demonstrated a 35% decline in overall complications and a significant reduction in mortality (57). The fact that the benefits of preoperative nutritional support are limited to those with a substantial degree of malnutrition is not terribly surprising and is an observation that recapitulates the results of trials that have included only patients with nonneoplastic disease. The same conclusion has been reached by meta-analyses (58,59). Thus, the clinician must establish an objective and practical means of stratifying patients into those who are moderately to severely malnourished, using algorithms such as those discussed in the previous section. Another critical point is that the ability of aggressive nutritional support to diminish perioperative complications is probably lost if it is deferred until after surgery. This has been observed in many trials, including those encompassing patients with gastric, pancreatic, and colorectal cancers (57,60).
The benefits of aggressive nutritional support in the preoperative period are not confined to TPN; provision of nutrients via an enteral approach is also of substantial benefit. The number of trials done with preoperative enteral support are far fewer and less compelling than those with TPN, but the existing studies indicate that preoperative enteral support conveys the same nutritional (61) and clinical (62) benefits to the malnourished patient as TPN. As was the case with TPN, in the absence of aggressive preoperative support, postoperative enteral nutrition seems to convey little advantage to the patient in the perioperative period (63), although individuals who receive a specialized immunomodulatory enteral formula appear to be an exception to this rule, an issue that is discussed in detail in the following section.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








