CHAPTER 19 Gastrointestinal Bleeding
The annual rate of hospitalization for any type of gastrointestinal (GI) hemorrhage in the United States is estimated to be 350 hospital admissions/100,000 population, with more than 1,000,000 hospitalizations annually.1 Approximately 50% of admissions for GI bleeding are for upper GI (UGI) bleeding (from the esophagus, stomach, duodenum), 40% are for lower GI (LGI) bleeding (from the colon and anorectum), and 10% are for obscure bleeding (from the small intestine).
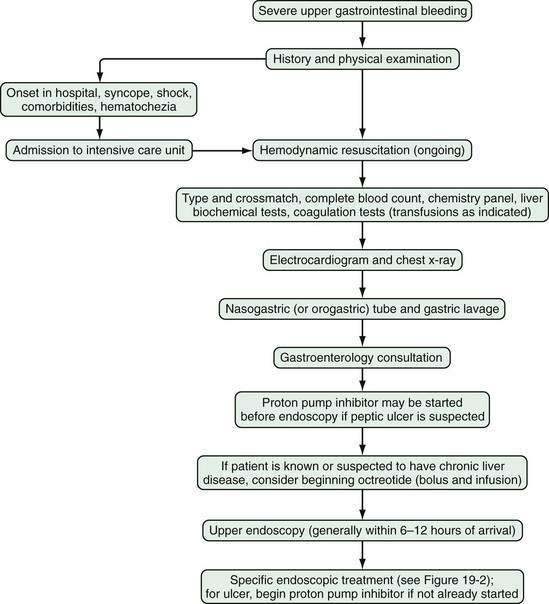
Severe gastrointestinal bleeding is defined as documented gastrointestinal bleeding (i.e., hematemesis, melena, hematochezia, or positive nasogastric lavage) accompanied by shock or orthostatic hypotension, a decrease in the hematocrit value by at least 6% (or a decrease in the hemoglobin level of at least 2 g/dL), or transfusion of at least two units of packed red blood cells. Most patients with severe gastrointestinal bleeding are admitted to the hospital for resuscitation and treatment. Overt bleeding implies visible signs of blood loss from the GI tract. Hematemesis is defined as vomiting of blood, which is indicative of bleeding from the esophagus, stomach, or duodenum. Hematemesis includes vomiting of bright red blood, which suggests recent or ongoing bleeding, and dark material (coffee-ground emesis), which suggests bleeding that stopped some time ago. Melena is defined as black tarry stool and results from degradation of blood to hematin or other hemochromes by intestinal bacteria. Melena can signify bleeding that originates from UGI, small bowel, or proximal colonic source. Melena generally occurs when 50 to 100 mL or more of blood is delivered into the GI tract (usually the upper tract), with passage of characteristic stool occurring several hours after the bleeding event.2,3 Hematochezia refers to bright red blood per rectum, and suggests active UGI or small bowel bleeding, or distal colonic or anorectal bleeding. Occult gastrointestinal bleeding refers to subacute bleeding that is not clinically visible. Obscure gastrointestinal bleeding is bleeding from a site that is not apparent after routine endoscopic evaluation with esophagogastroduodenoscopy (upper endoscopy) and colonoscopy, and possibly small bowel radiography. An algorithm for the initial management of acute, severe UGI bleeding is shown in Figure 19-1.
INITIAL ASSESSMENT AND MANAGEMENT OF ACUTE GASTROINTESTINAL BLEEDING
HISTORY
Initial assessment of the patient with acute GI bleeding includes medical history taking, obtaining vital signs, performing a physical examination, including a rectal examination, and nasogastric lavage. During history taking, patients should be questioned about risk factors and historical features that help identify diagnostic possibilities for the bleeding source (Table 19-1). Bleeding from a peptic ulcer should be suspected in patients with a history of an ulcer or those taking daily aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). Patients who have known or suspected liver disease or who are alcoholics should be suspected of bleeding related to portal hypertension. Patients with heavy alcohol intake, a feeding or chronic nasogastric tube, or a history of gastroesophageal reflux disease are at risk of erosive esophagitis. Patients who have had prior surgical repair of an abdominal aortic aneurysm should be considered to have a fistula from the graft to the duodenum until proven otherwise. Patients on an anticoagulant such as warfarin should be evaluated for the possibility of excessive anticoagulation. Prior radiation to the abdomen should raise the possibility of radiation enteritis or colitis. Weight loss suggests possible malignancy. Abdominal pain may suggest malignancy, inflammatory bowel disease, or ischemic colitis. A change in stool caliber suggests colon cancer or a colonic stricture. Chest pain or syncope suggests possible cardiovascular complications related to blood loss.
Table 19-1 Suspected Source of Gastrointestinal Bleeding as Suggested by a Patient’s History
| SUSPECTED SOURCE OF BLEEDING | PATIENT HISTORY |
|---|---|
| Nasopharynx | |
| Lungs | Hemoptysis |
| Esophageal ulceration | |
| Esophageal cancer | |
| Mallory-Weiss tear | |
| Cameron’s erosions | Large hiatal hernia |
| Esophageal or gastric varices or portal hypertensive gastropathy | |
| Gastric angiodysplasia | Chronic kidney disease |
| Peptic ulcer | |
| Gastric cancer | |
| Primary aortoenteric fistula | Prior severe acute unexplained bleeding |
| Secondary aortoenteric fistula | Prior abdominal aortic aneurysm surgical repair with synthetic graft |
| Ampulla of Vater | Recent endoscopic sphincterotomy |
| Bile ducts | Recent liver biopsy or cholangiography |
| Pancreatic ducts | Pancreatitis, pseudocyst |
| Recent pancreatography | |
| Small intestine malignancy | |
| Meckel’s diverticulum | Unexplained gastrointestinal bleeding since childhood |
| Small intestine or colon ulcerations | Use of aspirin or other nonsteroidal anti-inflammatory drug |
| Small intestine telangiectasias | |
| Small intestine angiodysplasia | Age > 60 years |
| Colonic diverticulosis | |
| Colonic neoplasia | |
| Ischemic colitis | |
| Ulcerative colitis | |
| Crohn’s disease | |
| Anal fissure | Hematochezia with anal pain |
| Hemorrhoids | |
| Postpolypectomy ulcer | |
| Colonic or small intestinal angioectasias | |
| Anastomotic ulceration | Prior intestinal surgical anastomosis |
| Radiation enteritis or proctitis | History of abdominal radiation therapy |
IBD, inflammatory bowel disease.
PHYSICAL EXAMINATION
On initial evaluation, physical examination should focus on the patient’s vital signs, with attention to signs of hypovolemia such as hypotension, tachycardia, and orthostasis. The abdomen should be examined for surgical scars, tenderness, and masses. Signs of chronic liver disease include spider angiomata, palmar erythema, gynecomastia, ascites, splenomegaly, caput medusae, and Dupuytren’s contracture. The skin, lips, and buccal mucosa should be examined for telangiectasias, which are suggestive of hereditary hemorrhagic telangiectasia (HHT), or Osler-Weber-Rendu disease. Pigmented lip lesions may suggest Peutz-Jeghers syndrome. Purpuric skin lesions may suggest Henoch-Schönlein purpura. Acanthosis nigricans may suggest underlying malignancy, especially gastric cancer. The patient’s feces should be observed to identify melena or maroon and red stool; however, the subjective description of stool color varies greatly among patients and physicians.4
LABORATORY STUDIES
Blood from the patient with acute GI bleeding should be sent for standard hematology, chemistry, liver biochemical, and coagulation studies and for typing and crossmatching for packed red blood cells. The hematocrit value immediately after the onset of bleeding may not reflect blood loss accurately because over 24 to 72 hours there is equilibration of red blood cells in the vascular space with extravascular fluid and hemodilution resulting from intravenous administration of saline.5 The mean corpuscular volume (MCV) is an important indicator of the chronicity of blood loss; an MCV lower than 80 fL suggests chronic GI blood loss and iron deficiency, which can be confirmed by the finding of low blood iron, total iron-binding capacity (TIBC), and ferritin levels. A low MCV and negative fecal occult blood test result raise the possibility of celiac disease. A high MCV (>100 fL) suggests chronic liver disease or folate or vitamin B12 deficiency. An elevated white blood cell count may occur in more than half of patients with UGI bleeding and has been associated with greater severity of bleeding.6 A low platelet count can contribute to the severity of bleeding and suggests chronic liver disease or a hematologic disorder.
The blood urea nitrogen (BUN) and serum creatinine levels can help assess the patient for hemoconcentration (elevated levels) or chronic kidney disease, which may lead to chronic anemia because of decreased erythropoietin production. In patients with UGI bleeding, the BUN level typically increases to a greater extent than the serum creatinine level because of increased intestinal absorption of urea after the breakdown of blood proteins by intestinal bacteria.7
CLINICAL DETERMINATION OF THE BLEEDING SITE
Presentation with hematemesis, coffee-ground emesis, or nasogastric lavage with return of a large amount of blood or coffee-ground emesis indicates an UGI source of bleeding. A small amount of coffee-ground material or pink-tinged fluid that clears easily may represent mucosal trauma from the nasogastric tube rather than active bleeding from an UGI source. A clear (nonbloody) nasogastric aspirate does not necessarily indicate a more distal GI source bleeding, because 16% of patients with actively bleeding UGI lesions have a clear nasogastric aspirate.8 The presence of bile in the nasogastric aspirate makes UGI bleeding unlikely but can be seen with an intermittently bleeding UGI source.
Melena generally indicates an UGI source but can be seen with small intestinal or proximal colonic bleeding. Hematochezia generally implies a colonic or anorectal source of bleeding unless the patient is hypotensive, which could indicate a severe, brisk UGI bleed with rapid transit of blood through the GI tract.4 Maroon-colored stool can be seen with an actively bleeding UGI source or a small intestinal or proximal colonic source.
HOSPITALIZATION
On the basis of the patient’s initial history, physical examination, and laboratory test results, the location of bleeding (upper or lower), suspected bleeding lesion, and severity of bleeding can be predicted. Patients with severe GI bleeding require hospitalization, whereas those who present with only mild acute bleeding (self-limited hematochezia or infrequent melena) and who are hemodynamically stable (not suspected to be volume depleted), have normal blood test results, and can be relied on to return to the hospital if symptoms recur may be candidates for semiurgent outpatient endoscopy rather than direct admission to the hospital.9,10 On the other hand, patients should be hospitalized in an intensive care unit if they have large amounts of red blood in the nasogastric tube or per rectum, have unstable vital signs, or have had severe acute blood loss that may exacerbate other underlying medical conditions. Patients who have had an acute GI bleed but are hemodynamically stable can be admitted to a monitored bed (step-down unit) or standard hospital bed, depending on their clinical condition.
Several small studies have suggested that urgent endoscopy performed in the emergency department in patients with suspected UGI bleeds can help determine optimal hospital placement; however, widespread implementation of this practice is unlikely.11,12
INITIAL MEDICAL THERAPY
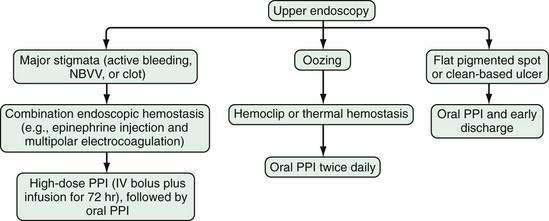
Administration of a proton pump inhibitor (PPI) is useful for reducing rebleeding rates in patients with peptic ulcer disease (see later). Starting a PPI in the emergency department or intensive care unit (ICU) before endoscopy is performed in patients with severe UGI bleeding has become a common practice but is still controversial.13 Several clinical studies and meta-analyses have shown that infusion of a high-dose PPI before endoscopy accelerates the resolution of endoscopic stigmata of bleeding in ulcers (see later) and reduces the need for endoscopic therapy but does not result in improved clinical outcomes in the transfusion requirement, rebleeding rate, need for surgery, or death rate.14–17 Patients with a strong suspicion of portal hypertension and variceal bleeding should be started empirically on intravenous octreotide (bolus followed by infusion [see later and Chapter 90]), which can reduce the risk of rebleeding to a rate similar to that associated with endoscopic therapy (Fig. 19-2; also see Fig. 19-1).18,19
ENDOSCOPY
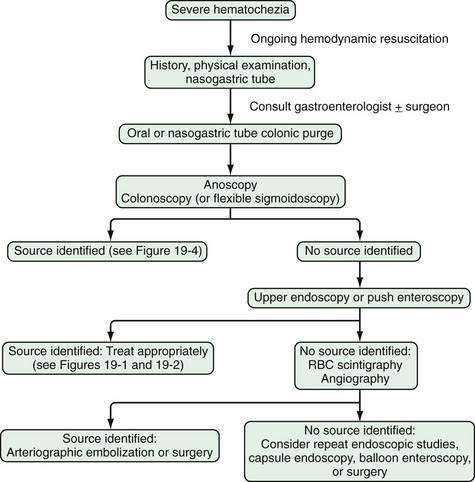
GI endoscopy will identify the bleeding site and permit therapeutic hemostasis in most patients with GI bleeding. Endoscopy should be done only when it is safe to do so and when the information obtained from the procedure will influence patient care. Ideally, the patient should be hemodynamically stable, with a heart rate of less than 100 beats/min and a systolic blood pressure higher than 100 mm Hg. Respiratory insufficiency, altered mental status, or ongoing hematemesis indicates the need for endotracheal intubation before emergency upper endoscopy to stabilize the patient and protect the airway. Coagulopathy and thrombocytopenia should be corrected with transfusions prior to endoscopy. Proper medical resuscitation will not only allow safer endoscopy, but also ensure a better diagnostic examination for lesions, such as varices, that are volume dependent and will allow more effective hemostasis because of the correction of coagulopathy (Figs. 19-3 and 19-4; also see Figs. 19-1 and 19-2).
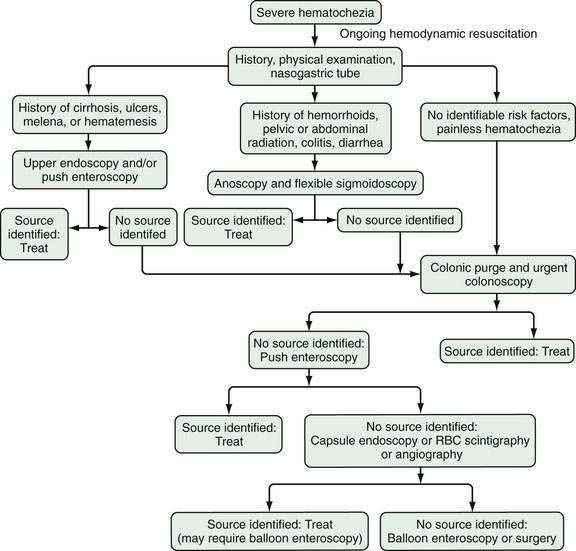
Figure 19-4. Algorithm for the management of severe hematochezia modified according to the patient’s history. RBC, red blood cell.
In patients with severe UGI bleeding, gastric lavage with a large (34-Fr) orogastric tube should be performed to evacuate blood and clots from the stomach to prevent aspiration and allow good endoscopic visualization. Special lavage systems can help remove blood rapidly. The intravenous administration of erythromycin (a gastric prokinetic agent) 30 to 90 minutes before upper endoscopy to induce gastric contraction and push blood from the stomach into the small intestine helps endoscopic visualization.20,21 Therapeutic single- or double-channel endoscopes with large-diameter suction channels should be used to allow quick removal of fresh blood from the GI tract during endoscopy. Additionally, a water pump can be used to irrigate target lesions through an accessory channel and dilute blood for suctioning, both of which facilitate visualization. Using iced saline lavage to prevent or decrease UGI bleeding is of no particular value and may impair coagulation and cause hypothermia. Gastric lavage with lukewarm tap water is as safe as lavage with sterile saline and much less expensive.
In patients with severe hematochezia and suspected active colonic bleeding, urgent colonoscopy can be undertaken after a rapid purge (see Figs. 19-3 and 19-4).22,23 Patients should receive 4 to 8 L of polyethylene glycol purge orally or via a nasogastric tube over four to six hours until the rectal effluent is clear of stool, blood, and clots. Additional polyethylene glycol bowel purge may be required in some patients, particularly those with active bleeding, severe constipation, or the onset of hematochezia in the hospital. Metoclopramide, 10 mg, may be given intravenously before the purge and repeated every four to six hours to facilitate gastric emptying and reduce nausea. In patients with severe or ongoing active hematochezia, urgent colonoscopy should be performed within 12 hours, but only after thorough cleansing of the colon. Patients with mild or moderate self-limited hematochezia should undergo colonoscopy within 24 hours of admission, and a colonic purge is also recommended in this situation to cleanse the colon thoroughly.
Patients with maroon stool in whom there is pretest uncertainty about the bleeding source should be considered for an urgent polyethylene bowel preparation as well. Colonoscopy immediately after push enteroscopy (see later), while the patient is still sedated, will expedite a patient’s care if push enteroscopy does not provide a diagnosis (and is also indicated for colon cancer screening in patients older than 50 years; Fig. 19-5).
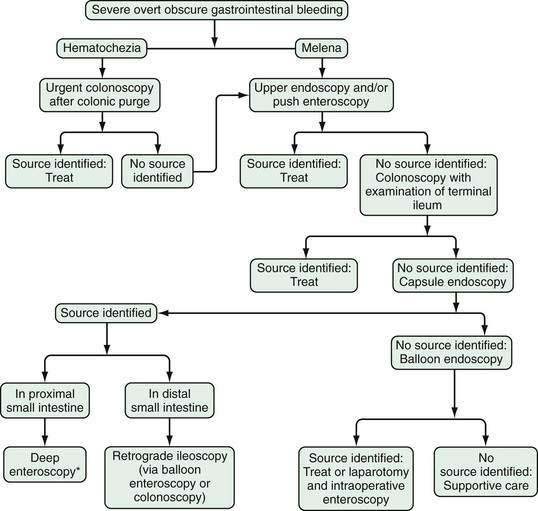
Wireless small bowel capsule endoscopy (or capsule endoscopy; see later) can be useful in patients with overt GI bleeding who have normal push enteroscopy and colonoscopy results and in whom a small bowel source of bleeding is suspected.24 Capsule endoscopy has the advantages of directly visualizing the small intestine to identify potential sources or active bleeding. Disadvantages are that the procedure takes eight hours to complete and additional time to download and review the images, does not permit therapeutic hemostasis, and is difficult to perform in inpatients because of limited availability of staff trained to place the capsule. A follow-up endoscopic procedure, such as single- or double-balloon enteroscopy or retrograde ileoscopy, may be indicated for definitive diagnosis and treatment if a focal bleeding site is found on capsule endoscopy.
Complications related to emergency endoscopy and endoscopic hemostasis may occur in up to 1% of patients, depending on the type of endoscopy and treatment performed.25,26 The most common complications include GI tract perforation, aspiration pneumonia, induced hemorrhage, an adverse medication reaction, hypotension, and hypoxia (see Chapter 40).
ENDOSCOPIC HEMOSTASIS
Thermal contact probes have been the mainstay of endoscopic hemostasis since the 1970s. These probes come in diameters of 7 and 10 Fr and in lengths that can fit through panendoscopes, enteroscopes, or colonoscopes. Contact probes can physically tamponade a blood vessel to stop bleeding and interrupt underlying blood flow, and thermal energy is then applied to seal the underlying vessel (coaptive coagulation). The most commonly used probe is a multipolar electrocoagulation (MPEC) probe, also referred to as a bipolar electrocoagulation probe, with which heat is created by current flowing between intertwined electrodes on the tip of the probe. Animal studies in which MPEC probes were used to stop bleeding in mesenteric vessels have shown that optimal coagulation occurs with low-power settings (12 to 16 W) applied for a moderate amount of time (8 to 10 seconds), with moderate pressure on the bleeding site.27 Heater probes can provide a predetermined amount of joules of energy, which does not vary with tissue resistance. Animal studies have shown that heater probes can effectively coagulate arteries up to 2 mm in diameter, a diameter considerably larger than most secondary or tertiary branches of arteries (usually 1 mm) found in resected bleeding human peptic ulcers.28,29 The main risk of using a thermal probe is perforation with excessive application of coagulation or pressure, especially in acute or nonfibrotic lesions. Thermal probes can also cause a coagulation injury that can make lesions larger and deeper and may induce delayed bleeding in patients with a coagulopathy.
Injection therapy is performed most commonly with the use of a sclerotherapy needle to inject epinephrine, diluted to a concentration of 1 : 10,000 or 1 : 20,000, submucosally into or around the bleeding site or stigma of hemorrhage (see later). The advantages of this technique are its wide availability, relatively low cost, and safety in patients with a coagulopathy. Additionally, it is associated with a lower risk of perforation and thermal burn damage than the thermal techniques. The disadvantage of epinephrine injection is that it is not as effective for definitive hemostasis as thermal coagulation, hemoclipping (see later), or combination therapy.30,31 Injection therapy can also be performed with a sclerosant, such as ethanolamine or alcohol, but these agents are associated with increased tissue damage and other risks.
Endoscopic hemoclips (or clips) have been available since 1974, and have become popular as technical improvements have been introduced.32 Hemoclips serve to apply mechanical pressure to a bleeding site, as is done with surgical clips or sutures. Endoscopic hemoclips differ from surgical clips, however, in that they do not have as much compressive strength, and the currently available clips do not close completely but leave a small space between the prongs. Animal studies have shown that the first-generation hemoclips could not stop bleeding in vessels larger than a diameter of 1 mm.33 Subsequent hemoclips have been larger and stronger and have had a grasp and release mechanism that improves endoscopic deployment and hemostasis. By not causing significant thermal damage, hemoclips are especially useful for patients with malnutrition or coagulopathy.34 Nevertheless, hemoclips can also be difficult to deploy depending on the location of the bleeding site, the degree of fibrosis of the underlying lesion, and limitations to endoscopic access.
Band ligation is a technique in which mucosal (with or without submucosal) tissue is suctioned into a cap placed on the end of the endoscope, and a rubber band is rolled off the cap and over the lesion to compress its base. This technique is widely used for the treatment of esophageal varices (see Chapter 90) and occasionally can be used for other bleeding lesions. An advantage of band ligation is that it is relatively easy to perform; however, a disadvantage is that sufficient mucosa must be suctioned into the cap for ligation to be successful. Depending on the manufacturer, some band ligation devices can only fit on diagnostic endoscopes, and switching from a larger therapeutic endoscope to a smaller diagnostic endoscope during a case is time-consuming and inefficient.
RADIOLOGIC IMAGING
Angiography may be used to diagnose and treat severe bleeding, especially when the cause cannot be determined by upper and lower endoscopy. Angiography generally is diagnostic of extravasation into the intestinal lumen only when the arterial bleeding rate is at least 0.5 mL/min.35 The sensitivity of mesenteric angiography is 30% to 50% (with higher sensitivity rates for active GI bleeding than for recurrent acute or chronic occult bleeding), and the specificity is 100%.36 An advantage of angiography is that it permits therapeutic intra-arterial infusion of vasopressin or transcatheter embolization for hemostasis if active bleeding is detected, without the need for bowel cleansing. Nevertheless, the rate of major complications, including hematoma formation, femoral artery thrombosis, contrast dye reactions, acute kidney injury, intestinal ischemia, and transient ischemic attacks, is 3%.37 Another disadvantage of angiography is that it usually does not identify the specific cause of bleeding, only its location.
Radionuclide imaging is occasionally helpful for patients with unexplained GI bleeding, although it is used less frequently now than in the past because of the widespread use of endoscopy and lack of availability of nuclear medicine services for emergencies, particularly at night and on weekends. Radionuclide imaging can be performed relatively quickly and may help localize the general area of bleeding and thereby guide subsequent endoscopy, angiography, or surgery. The technique involves injecting a radiolabeled substance intravenously into the patient’s bloodstream and then performing serial scintigraphy to detect focal collections of radiolabeled material. Radionuclide imaging has been reported to detect bleeding at a rate of 0.04 mL/min.38 The two tracers used for radionuclide imaging for bleeding (bleeding scans) are technetium sulfur colloid and technetium pertechnetate–labeled autologous red blood cells. Technetium sulfur colloid is cleared rapidly from the bloodstream and is therefore useful for identifying acute, active bleeding. Technetium pertechnetate–labeled red blood cells remain in the circulation for up to 24 hours and therefore can be used for repeated scanning in patients with intermittent bleeding. A comparative study of patients with suspected GI bleeding has found technetium pertechnetate–labeled red blood cell scans to be more sensitive, specific, and accurate than technetium sulfur colloid scans.39
The overall rate of a diagnostic radionuclide scan is approximately 45%, with a 78% accuracy rate in the localization of the true bleeding site.40,41 Up to 25% of bleeding scans suggest a site of bleeding that proves to be incorrect.41–43 The rate of true-positive scans is higher for active bleeding with hemodynamic instability than for less severe bleeding.44 The most common reason for a false-positive result is rapid transit of luminal blood, so that labeled blood is detected in the colon even though it originated from a more proximal site in the GI tract. Caution is recommended in using the results of delayed scans to localize and target lesions for resective surgery.45
Technetium pertechnetate scintigraphy can identify ectopic gastric mucosa in a Meckel’s diverticulum. This diagnosis should be considered in a pediatric or young adult patient with unexplained GI bleeding. The positive predictive value, negative predictive value, and overall accuracy of a so-called Meckel’s scan has been reported to be higher than 90% in young patients.46,47 In patients older than 25 years, however, Meckel’s scans are much less sensitive (<50%). Caution is recommended in interpreting a negative Meckel’s scan result in an adult patient with GI bleeding.48
Conventional radiographic imaging is usually not needed in a patient with GI bleeding but occasionally may provide some important information. In patients with a prior abdominal aortic aneurysm repair and graft, computed tomography (CT) with intravenous contrast can identify inflammation between the graft and duodenum and thus suggest graft fistulization into the duodenum.49 In selected patients, an abdominal CT scan can also identify a mass lesion, such as an intra-abdominal tumor, or small bowel abnormalities that may suggest a cause of bleeding. Advances in CT scan technology have permitted CT enterography and CT angiography to be performed, with promising results.50,51
UPPER GASTROINTESTINAL BLEEDING
EPIDEMIOLOGY
UGI bleeding is a common medical emergency that accounts for more than 500,000 hospital admissions each year, or approximately 170 patients/100,000 population/year.1 Of the potential causes of severe UGI bleeding, peptic ulcer is the most common, accounting for approximately 40% of cases (Table 19-2).52,53 Despite advances in medical therapy, ICU care, endoscopy, and surgery, the mortality rate of 5% to 10% for severe UGI bleeding has not changed since the 1970s.1,52–56 The lack of decline in the mortality rate may be explained by an increase in the proportion of older patients with GI bleeding, who may die as a result of worsening of other medical conditions rather than from exsanguination, and an increase in the number of patients with cirrhosis and variceal bleeding.
Table 19-2 Causes of Severe Upper Gastrointestinal Bleeding in the UCLA CURE Database
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree