CHAPTER 43 Gastroesophageal Reflux Disease
EPIDEMIOLOGY
On the basis of symptoms, GERD is common in Western countries. In a nationwide population-based study by the Gallup Organization in the United States, 44% of the respondents reported heartburn at least once a month.1 More convincing data were obtained from a mailing of 2200 validated self-report questionnaires to a predominantly white population living in Olmsted County, Minnesota.2 The prevalence of heartburn and acid regurgitation in the past year was 42% and 45%, respectively. Symptoms that occurred at least weekly were reported by 20% of respondents, with an equal gender distribution across all ages. Most subjects reported their heartburn as being moderately severe, with a duration of 5 years or more, and only 5.4% had seen a physician for their reflux symptoms within the past year. More varying prevalence rates for symptomatic GERD have been reported from Europe, ranging from 5% in Switzerland to 27% in Finland.3
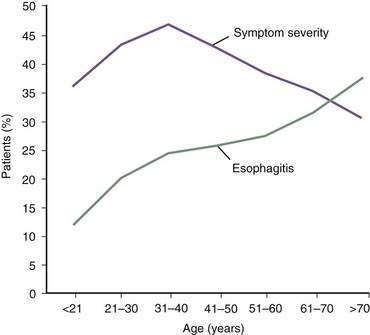
In contrast, the true prevalence of esophagitis is very difficult to define because healthy subjects rarely undergo upper endoscopy. Studies suggest that 7% of Americans have erosive esophagitis, whereas European studies identify prevalence rates ranging from 2% to 10%.4 GERD affects nearly equal proportions of men and women, but a male predominance occurs in esophagitis and Barrett’s esophagus.4 Increasing age is an important factor in the prevalence of GERD complications, probably the result of cumulative acid injury over time to the esophagus (Fig. 43-1).5,6
The prevalence of GERD only recently has been studied in multiracial populations. In a cross-sectional survey among employees at a Houston Veterans Affairs hospital, the prevalence of heartburn was similar (23% to 27%) across ethnic groups including African Americans, Hispanics, Asians, and whites. However, African Americans had significantly less esophagitis than whites (24% versus 50%) for the same severity of symptoms (weekly or more).7 A study from Boston reviewed endoscopic reports from nearly 2500 consecutive patients, finding complicated GERD in 12% of white patients, 3% of African American patients, and 2% of Asian patients.8
The prevalence of GERD is relatively low among residents of Africa and Asia. For example, a cross-sectional study in Singapore reported prevalence rates for reflux symptoms of 7.5% in Indians, 0.8% in Chinese, and 3% in Malays.9 There have been exceptions such as the remarkable increase in the frequency of reflux symptoms seen in Japan and Singapore.10 An endoscopic, population-based study from South Korea encompassing more than 25,000 individuals, found the prevalence of erosive esophagitis to be 8%, whereas nonerosive reflux disease occurred in 4% of examined individuals.11 More than 90 % of subjects with erosions had mild disease, consistent with previous endoscopic studies from Asia. A recent systematic review that summarized trend data from longitudinal population-based studies performed in Asia failed to demonstrate an increase in prevalence over the past decade.12 Possible reasons for the lower GERD prevalence include low dietary fat; low body mass index (BMI); and lower gastric acid output, possibly related to Helicobacter pylori infection.11,13
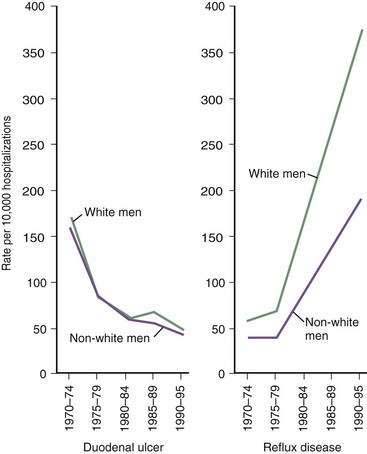
The prevalence of GERD has been increasing in Western countries over the past 30 years.14 El Serag and Sonnenberg observed opposing time trends in the prevalence of peptic ulcer disease and GERD in the United States. Rates of duodenal ulcer fell between 1970 and 1995, while the prevalence of GERD and esophageal adenocarcinoma rose significantly (Fig. 43-2).15 The authors speculated that the decreasing prevalence of H. pylori may be playing a contributory role to the increasing prevalence of GERD in these regions. Recent data suggest that many patients with H. pylori–induced gastritis have involvement of the antrum and corpus, decreasing parietal cell mass, reducing acid secretion, and elevating gastric pH.13 This may have a protective effect on the esophageal mucosa in patients susceptible to GERD.
An additional explanation for an increased prevalence of GERD in Western populations is the epidemic increase in obesity.16 In obese individuals (defined as a body mass index ≥30), epidemiologic studies suggest the prevalence of GERD is considerably higher than in the nonobese population.4,5,16 Jacobson and associates looked at the participants in the Nurses’ Health Study and found a nearly linear increase in the adjusted odds ratio for reflux symptoms for each BMI stratum.17 Interestingly, even for those participants with a normal BMI (22.5 to 24.9 kg/m2), the risk was elevated relative to a control group having a BMI in the range of 20 to 22.4 kg/m2.17 A study from the Houston VA Medical Center found a linear relationship between BMI and weekly symptoms of heartburn or regurgitation.18 Data from large population-based studies in England and Germany have been similar.19,20 A Norwegian study suggested that the odds of developing GERD were higher in obese subjects, and the risk was greater in obese women compared with male participants.21 In contrast, a nationwide case-control study from Sweden, consisting primarily of older adult men, failed to find an association between obesity and GERD.22
Central adiposity, as measured by the waist-to-hip ratio, may be more important than BMI in the pathogenesis of GERD. A large study from the Kaiser Permanente health system found a significant relationship between increased abdominal diameter and reflux symptoms independent of BMI.23 Similarly, El-Serag and colleagues found that the relationship between increasing BMI and increased acid exposure in the distal esophagus was primarily explained by the subject’s waist circumference.24
Obesity appears to be associated with complications related to long-standing GERD such as erosive esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma.18,25–27 In a Swedish case-control study, researchers identified an association between esophageal adenocarcinoma and an individual’s BMI 20 years prior to the development of malignancy.28 Another case-control study demonstrated that central adiposity rather than BMI was associated with the presence of Barrett’s esophagus, particularly long-segment disease.29
Several mechanisms have been proposed to explain the association between obesity and GERD. These include an increased prevalence of esophageal motor disorders, diminished lower esophageal sphincter pressure, increased prevalence of hiatal hernia, and increased intragastric pressure (particularly with central obesity).30 In addition, visceral fat is metabolically active and produces a variety of cytokines including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which may affect the function of the lower esophageal sphincter.
Along with environmental factors, the epidemiology of GERD may be affected by genetics. Family clustering of GERD and its complications, especially Barrett’s esophagus, have been reported.31,32 Especially exciting are the observations from two large case-control studies of twins from the United States and Sweden33,34 suggesting that genetic liability for GERD, as defined by frequent symptoms, is in the range of 30% to 45%. Although one group defined a locus on chromosome 13 associated with severe pediatric GERD,35 this has not been confirmed by other pediatric researchers36 and not yet evaluated in adults. The genetic mechanisms are unknown but may be related to a smooth muscle disorder associated with hiatal hernia, reduced lower esophageal sphincter (LES) pressure, and impaired esophageal motility.32
HEALTH CARE IMPACT
Although rarely a cause of death, GERD is associated with considerable morbidity and complications, such as esophageal ulceration (5%), peptic stricture (4% to 20%), and Barrett’s esophagus (8% to 20%).5 Not surprisingly, the burden of GERD on health care is great. In 2004, GERD was by far the most common digestive disease diagnosis during ambulatory care visits, constituting 17.5% of all GI diagnoses. There were 6 or more outpatient visits with a GERD diagnosis listed per 100 people in the United States.37 GERD was the 10th most common inpatient GI diagnosis, with an estimated total number of discharges of 95,000 per year, a two day median length of stay, and median charges of $8060.38 In summary, GERD was the second most costly GI disease in 2004, behind liver disease, with total direct and indirect costs of nearly $12.6 billion. More than 60 million prescriptions for the treatment of GERD were estimated to be filled at retail pharmacies in 2004, representing 48% of all prescriptions for GI disorders and more than 50% of their costs. The large majority of prescriptions and their costs were for proton pump inhibitors, which were the five most commonly prescribed and costliest GI medications.37 In 2004, two PPIs (lansoprazole and esomeprazole) were the second and fourth, respectively, top selling drugs of all classes in the United States.
A recent economic survey from Germany reported that 6% of individuals with established GERD missed at least one day of work per year due to this disorder. Sixty-one percent of these patients visited their physician at least once in the previous year and 2% were hospitalized specifically for GERD.39 They estimated direct and indirect costs of approximately $600 per patient per year.
Furthermore, GERD as a chronic disease significantly impairs quality of life. Compared with other chronic medical conditions, this impairment is similar to, or even greater than, that from arthritis, myocardial infarction, heart failure, or hypertension.40 GERD comorbidities are common and include irritable bowel syndrome and psychological distress in 36% and 41% of patients, respectively.41 These comorbidities potentiate the negative effect on quality of life seen with GERD, and affect the response to treatment with proton pump inhibitors.
PATHOGENESIS
ANTIREFLUX BARRIERS
The first tier of the three-tiered esophageal defense against acid damage, the antireflux barriers, is an anatomically complex region including the intrinsic lower esophageal sphincter (LES), diaphragmatic crura, the intra-abdominal location of the LES, the phrenoesophageal ligaments, and the acute angle of His (Fig. 43-3).
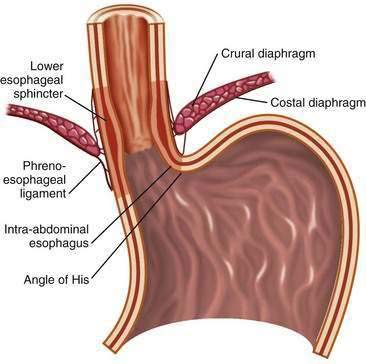
Figure 43-3. Anatomy of the gastroesophageal junction illustrating the major elements of the antireflux barrier.
The LES involves the distal 3 to 4 cm of the esophagus and at rest is tonically contracted.42 It is the major component of the antireflux barrier, being capable of preventing reflux even when completely displaced from the diaphragmatic crura by a hiatal hernia.43 The proximal portion of the LES is normally 1.5 to 2 cm above the squamocolumnar junction, whereas the distal segment, about 2 cm in length, lies within the abdominal cavity. This location maintains gastroesophageal competence during intra-abdominal pressure excursions. Resting LES pressure ranges from 10 to 30 mm Hg with a generous reserve capacity because only a pressure of 5 to 10 mm Hg is necessary to prevent GER.44 The LES maintains a high-pressure zone by the intrinsic tone of its muscle and by cholinergic excitatory neurons.45,46 There is considerable diurnal variation in basal LES pressure; it is lowest after meals and highest at night, and large increases occur with phase III of the migrating motor complex. It is also influenced by circulating peptides and hormones, foods (particularly fat), as well as a number of drugs (Table 43-1) (see also Chapter 48).
Table 43-1 Modulators of Lower Esophageal Sphincter Pressure
| INCREASE LES PRESSURE | DECREASE LES PRESSURE | |
|---|---|---|
| Hormones/peptides | Gastrin | Secretin |
| Motilin | Cholecystokinin | |
| Substance P | Somatostatin | |
| VIP | ||
| Neural agents | α-Adrenergic agonists | α-Adrenergic antagonists |
| β-Adrenergic antagonists | β-Adrenergic agonists | |
| Cholinergic agonists | Cholinergic antagonists | |
| Foods | Protein | Fat |
| Chocolate | ||
| Peppermint | ||
| Other factors | Histamine | Theophylline |
| Antacids | Prostaglandins E2 and I2 | |
| Metoclopramide | Serotonin | |
| Domperidone | Meperidine | |
| Cisapride | Morphine | |
| Prostaglandin F2α | Dopamine | |
| Baclofen | Calcium channel blockers | |
| Diazepam | ||
| Barbiturates |
LES, lower esophageal sphincter; VIP, vasoactive intestinal peptide.
The LES lies within the hiatus created by the right crus of the diaphragm and is anchored by the phrenoesophageal ligaments, which insert at the level of the squamocolumnar junction (see Fig. 43-3). Developmentally, the crural diaphragm arises from the dorsal mesentery of the esophagus and is innervated separately from the costal diaphragm. It is inhibited by esophageal distention, vomiting, and during transient LES relaxations (tLESRs), but not during swallowing. The crural diaphragm provides extrinsic squeeze to the intrinsic LES, contributing to resting pressure during inspiration and augmenting LES pressure during periods of increased abdominal pressure, such as with coughing, sneezing, or bending.47 Crural contractions impose rhythmic pressure increases of about 5 to 10 mm Hg on the LES pressure recording. During deep inspirations and some periods of increased abdominal straining, these changes may lead to pressures of 50 to 150 mm Hg.48
The oblique entrance of the esophagus into the stomach creates a sharp angle on the greater curve aspect of the gastroesophageal junction, the angle of His. This angle has been shown in cadavers to create a flap valve effect that contributes to gastroesophageal junction competency.49
MECHANISMS OF REFLUX
Transient Lower Esophageal Sphincter Relaxations
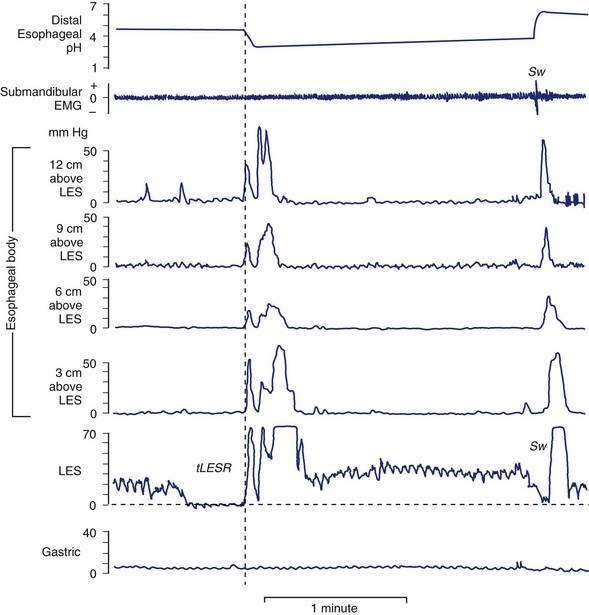
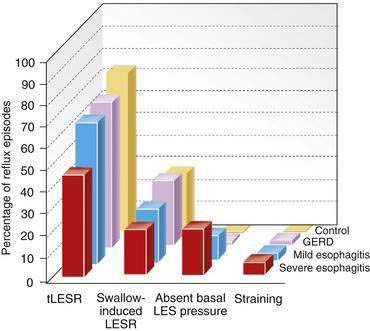
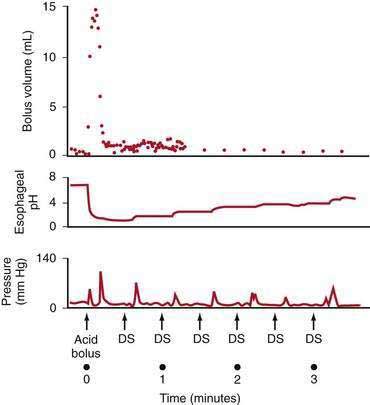
tLESRs are the most frequent mechanism for reflux in patients with healthy sphincter pressures. Figure 43-4 illustrates a transient LESR and highlights differences from swallow-induced LESRs. tLESRs occur independently of swallowing, are not accompanied by esophageal peristalsis, persist longer (>10 seconds) than swallow-induced LESRs, and are accompanied by inhibition of the crural diaphragm.50 tLESRs account for nearly all reflux episodes in healthy subjects and 50% to 80% of episodes in GERD patients, depending on the severity of associated esophagitis (Fig. 43-5).51 However, one study suggests that low basal LES pressure, rather than tLESRs, may be the primary mechanism of GER in patients with nonreducible hiatal hernias.52
tLESRs are not always associated with GER. In normal subjects 40% to 60% of tLESRs are accompanied by reflux episodes, compared with 60% to 70% in GERD patients.45,51,53 Possible factors determining whether reflux occurs include abdominal straining, presence of a hiatal hernia, degree of esophageal shortening, and duration of tLESRs. The dominant stimulus for tLESR is distention of the proximal stomach by either food or gas,54,55 which is not surprising given that a tLESR is the mechanism of belching. More varying stimuli are fat, stress, and subthreshold (for swallowing) stimulation of the pharynx.49 Various drugs may impair tLESRs including cholecystokinin A (CCK-1) receptor antagonists, anticholinergic drugs, morphine, somatostatin, nitric oxide inhibitors, 5-hydroxytryptamine (5-HT)3 antagonists, and γ-aminobutyric acid (GABAB) agonists.56
Evidence indicates that tLESRs are mediated through vagal pathways.54 Gastric distention activates mechanoreceptors (intraganglionic lamellar endings) adjacent to the gastric cardia, sending signals to the brainstem center via vagal afferent pathways.57 The structured sequence of motor events including LESR, crural diaphragm inhibition, and secondary esophageal peristalsis suggests that this process occurs in a programmed manner, probably controlled by a pattern generator within the vagal nuclei. The motor arm is the vagus nerve sharing common elements with swallow-induced LESR.56
Swallow-Induced Lower Esophageal Sphincter Relaxations
About 5% to 10% of reflux episodes occur during swallow-induced LESRs. Most episodes are associated with defective or incomplete peristalsis.53 During a normal swallow-induced LESR, reflux is uncommon because (1) the crural diaphragm does not relax, (2) the duration of LESR is relatively short (5 to 10 seconds), and (3) reflux is prevented by the oncoming peristaltic wave (see Fig. 43-4). Reflux during swallow-induced LESRs is more common with a hiatal hernia. This may be due to the lower compliance of the esophagogastric junction in hernia patients, permitting it to open at pressures equal to or lower than intragastric pressure, thereby allowing reflux of gastric juices accumulating in the hiatal hernia.58,59
Hypotensive Lower Esophageal Sphincter Pressure
GER can occur in the context of a hypotensive LES by either strain-induced or free reflux.44,51 Strain-induced reflux occurs when a relatively hypotensive LES is overcome and “blown open” by an abrupt increase in intra-abdominal pressure from coughing, straining, or bending over. This type of reflux is unlikely when the LES pressure is greater than 10 mm Hg. Free reflux is characterized by a fall in intraesophageal pH without an identifiable change in intragastric pressure, usually occurring when LES pressure is less than 5 mm Hg. Reflux due to a low or absent LES pressure is uncommon. Mostly it occurs in patients with severe esophagitis and may account for up to 25% of reflux episodes (see Fig. 43-5); it rarely occurs in patients without esophagitis.45,51,60 The mechanisms responsible for idiopathic low LES pressure (i.e., not part of a systemic disease such as scleroderma) are poorly understood. The presence of a hiatal hernia reduces the pressure measured in the LES due to losing the intrinsic support of the crural diaphragm.44 Some LES weakness may be secondary to esophagitis impairing the excitatory cholinergic pathways to the LES. Induction of experimental esophagitis in cats attenuates the release of acetylcholine and lowers LES pressures—changes that are reversible on healing of the esophagitis.60 However, healing of esophagitis in humans is rarely accompanied by an increase in LES pressure.61
HIATAL HERNIA
The contribution of the hiatal hernia to GERD is controversial. Opinion has shifted widely from one that virtually equated hiatal hernia with reflux disease to one that denied it a causal role. Epidemiologic and physiologic data confirm the importance of the hiatal hernia in patients with more severe esophagitis, peptic stricture, or Barrett’s esophagus.62 Hiatal hernia occurs in 54% to 94% of patients with reflux esophagitis, a rate strikingly higher than that in the healthy population.63 Two studies have also found that in individuals with reflux symptoms, the presence of hiatal hernia confers a significantly increased risk of erosive esophageal injury.64
The hiatal hernia impairs LES function through several mechanisms, as well as impairing esophageal acid clearance (Fig. 43-6).65 Reflux is worse in patients having a “nonreducible” as opposed to a “reducible” hiatal hernia. Nonreducing hernias are those in which the gastric rugal folds remain above the diaphragm between swallows.62 Statistical modeling has revealed a significant interaction between hiatal hernia and LES pressure, such that the likelihood of GER is increased as basal LES pressure decreases, an effect substantially amplified by the presence of a hernia and as the hernia size increases.43
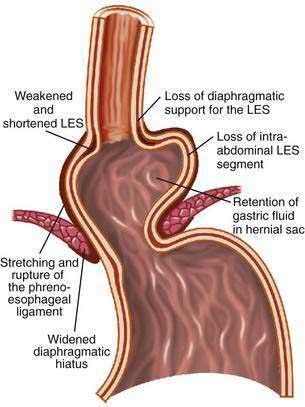
Figure 43-6. Schematic diagram showing the effect of a hiatal hernia on the antireflux barrier. LES, lower esophageal sphincter.
Displacement of the LES from the crural diaphragm into the chest reduces basal LES pressure and shortens the length of the high-pressure zone primarily due to the loss of the intra-abdominal LES segment.62 Hiatal hernia eliminates the increase of LES pressure that occurs during straining and increases tLESRs during gastric distention with gas.65,66 Large, nonreducible hernias also impair esophageal acid clearance because of an increased tendency for reflux to occur from the hernia sac during swallow-induced LESRs.53 Finally, an alteration of esophagogastric junction compliance, especially in GERD patients with hiatal hernia, has been identified.59 For the same degree of intragastric pressure, the esophageal junction opens at a lower pressure and the cross-sectional area is greater and more symmetrical as intragastric pressure increases. These changes in compliance simulated a 10-fold increase in air and 6-fold increase in liquid reflux across the esophageal junction.
The etiology of a hiatal hernia remains unclear. Familial clustering of GERD suggests the possibilities of an inherited smooth muscle disorder. Animal studies propose that reflux itself causes esophageal shortening promoting the development of a hiatal hernia.67 Other studies find an association with obesity68 and heavy lifting,69 raising the possibilities that over time chronic intra-abdominal stressors may weaken the esophageal hiatus, causing the development of a hiatal hernia. This theory is attractive as it helps to reconcile the increased prevalence of hiatal hernias as the population grows older.63
ESOPHAGEAL ACID CLEARANCE
Volume Clearance
Esophageal peristalsis clears acid volume in the upright and supine positions but is inoperative during deep rapid-eye-movement (REM) sleep. Helm and colleagues70 showed that one or two primary peristaltic contractions completely clear a 15-mL fluid bolus from the esophagus (Fig. 43-7). Primary peristalsis is elicited by swallowing. Secondary peristalsis, initiated by esophageal distention from acid reflux, is much less effective in clearing the refluxate, thus offering only an ancillary protective role.
Peristaltic dysfunction (i.e., failed peristaltic contractions and hypotensive [<30 mm Hg] peristaltic contractions that incompletely empty the esophagus) increases in frequency with the severity of esophagitis. Kahrilas and colleagues71 found that the prevalence of peristaltic dysfunction rose from 25% in individuals with mild esophagitis to more than 50% in patients with severe esophagitis. Whether esophagitis per se leads to peristaltic dysfunction or whether an underlying smooth muscle motility disorder of the esophagus predisposes to the development of reflux disease is not clear. Animal studies have found that esophageal dysmotility associated with active esophagitis is reversible, but esophageal dysmotility associated with stricture or extensive fibrosis is irreversible.60 Clinical observations suggest that impaired motor function does not revert to normal following either effective medical or surgical therapies.61
Gravity contributes to bolus clearance when reflux occurs in the upright position. At night when supine, this mechanism is not operative unless the head of the bed is elevated. This important lifestyle change markedly improves acid clearance time and is most beneficial in patients with aperistalsis (i.e., scleroderma).72
Salivary and Esophageal Gland Secretions
Saliva is the second essential factor required for normal esophageal acid clearance. Compared with gastric acid, saliva is a weak base with a pH of 6.4 to 7.8.73 Although saliva is ineffective in neutralizing large acid volumes (5 to 10 mL), it easily neutralizes the small amount of acid remaining in the esophagus after several peristaltic contractions (see Fig. 43-7).70 The importance of saliva is supported by observation that increased salivation induced by oral lozenges or bethanechol significantly decreases acid clearance time. In contrast, suction aspiration of saliva markedly prolongs acid clearance, despite the presence of normal peristaltic contractions.73
Modulation of salivation may contribute to GERD. Decreased salivation during sleep is the reason that nocturnal reflux episodes are associated with markedly prolonged acid clearance times.74 Xerostomia (see Chapter 22) is associated with prolonged esophageal acid exposure and esophagitis.75 Cigarette smoking promotes GER. Originally attributed to nicotine’s effect on lowering LES pressure, cigarette smokers also have prolonged esophageal acid clearance times due to hyposalivation.76 Finally, the esophagosalivary reflex is impaired in patients with reflux esophagitis and individuals with strictures.77 This is a vasovagal reflex demonstrated by perfusing acid into the esophagus, which stimulates salivation. This reflex explains the symptoms of water brash (copious salivation) observed in some reflux patients.
In addition to saliva, the aqueous bicarbonate-rich secretions of the esophageal submucosal glands dilute and neutralize residual esophageal acid.78 Acid refluxing into the esophageal lumen stimulates these glands and helps neutralize the acid, even if swallowing does not occur.79
Tissue Resistance
Although clearance mechanisms minimize acid contact time with the epithelium, even healthy subjects have acid reflux during the day and sometimes at night. Nevertheless, only a few subjects experience symptomatic GER and even fewer suffer GERD. This is due to a third tier for esophageal defense, known as tissue resistance. Conceptually, tissue resistance can be subdivided into pre-epithelial, epithelial, and postepithelial factors, which act together to minimize mucosal damage from the noxious gastric refluxate.80
The pre-epithelial defense in the esophagus is poorly developed. There is neither a well-defined mucous layer nor buffering capacity by the surface cells to secrete bicarbonate ions into the unstirred water layer. This results in a lumen-to-surface pH gradient in the esophagus of only 1 : 10, in contrast with the stomach and duodenum, where the gradient can range from 1 : 1000 to 1 : 10,000.81
The epithelial defenses consist of structural and functional components. Structural components include the cell membranes and intercellular junctional complexes of the esophageal mucosa. As reviewed in Chapter 41, this structure is a 25- to 30-cell-thick layer of nonkeratinized squamous epithelium functionally divided into a proliferating basal cell layer (stratum basalis), a midzone layer of metabolically active squamous cells (stratum spinosum), and a 5- to 10-cell-thick layer of dead cells (stratum corneum). The esophageal mucosa is a relatively “tight” epithelium that resists ionic movement at the intercellular, as well as the cellular, level as the result of tight junctions and the matrix of lipid-rich glycoconjugates in the intercellular space.82 Luminal acid attacks the epithelial defenses by damaging the intercellular junction, allowing hydrogen ions to enter and acidify the intercellular space. As documented by transmission electron microscopy, the intercellular spaces expand and eventually the buffering capacity of this space is overwhelmed, leading to acidification of the adjacent cytosol via the basolateral membrane.80 The functional components of tissue resistance include the ability of the esophageal epithelium to buffer and extrude hydrogen ions. Intracellular buffering is accomplished by negatively charged phosphates and proteins, as well as bicarbonate ions. When the mucosal buffering capacity is exceeded and intracellular pH falls, the epithelium has the capacity to actively remove or neutralize H+. This is possibly by the action of two transmembrane proteins, one an Na+/H+ exchanger and the other an Na+-dependent Cl−/HCO3− exchanger.83,84 After reflux-induced cell acidification, these transporters restore the intracellular pH to neutrality by exchanging H+ for extracellular Na+ or by exchanging Cl− for extracellular HCO3−, respectively. Additionally, esophageal cells contain within their membrane an Na+-independent Cl−/HCO3− exchanger that extrudes HCO3− from the cytoplasm when the intracellular pH is too high.83 When the epithelial cells are no longer able to maintain intracellular pH, they lose their ability to volume regulate, edema occurs, balloon cells develop, and cell death follows. Additional contributors to the epithelial defense include salivary epidermal growth factor, transforming growth factor-α, and prostaglandin E2. These factors enhance epithelial cell turnover, enhance esophageal mucin production, and modulate bicarbonate secretion.85
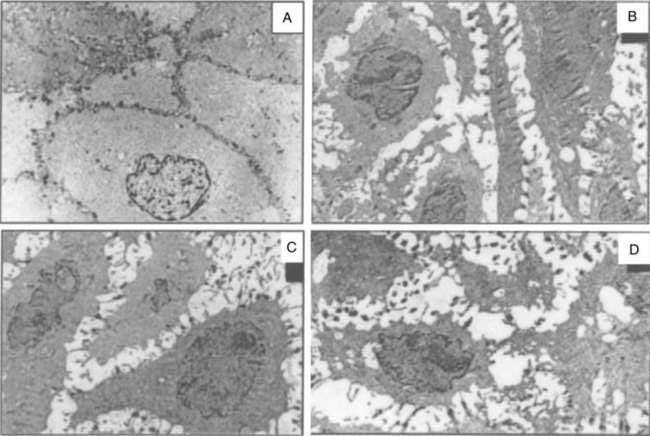
Data suggest that dilated intercellular spaces are the earliest markers of esophageal epithelial cellular damage (Fig. 43-8). These alterations arise with exposure to acid and pepsin during gastroesophageal reflux, but the exact pathway of damage to the intercellular junctions remains unclear and seems to be multifactorial.86 Other noxious contents of the refluxate, such as bile acids, are harmful, and dilated intercellular spaces can be induced by acute psychological stress.87 Dilated intercellular spaces can be assessed quantitatively with electron microscopy (EM), but they also are recognizable with light microscopy (LM). In studies by Calabrese and colleagues,88,89 all controls had intercellular spaces less than 1.69 µm. Symptomatic patients had a mean intercellular space value and a mean value of the maximum dilated intracellular space at least three times greater than controls. Statistical differences were not observed between esophagitis patients and nonerosive GERD patients. The authors speculated that increased paracellular permeability could partly explain the development of heartburn in the absence of overt esophagitis. This hypothesis is supported by the presence of sensory neuron receptors within the intercellular space, only a few cell layers from the esophageal lumen.90 Importantly, aggressive acid inhibition with proton pump inhibitors leads to complete resolution of the dilated intercellular spaces in nearly all patients over three to six months. These changes correlated closely with the resolution of heartburn.89
The postepithelial defense is provided by the esophageal blood supply. Blood flow delivers oxygen, nutrients, and bicarbonate and removes H+ and CO2, thereby maintaining normal tissue acid-base balance. Blood flow to the esophageal mucosa increases in response to the stress of luminal acid.91 Cellular injury also stimulates cell proliferation, which results in thickening of the basal cell layer of the epithelium. Unlike the stomach, where superficial mucosal injury can be repaired in hours, the esophagus repairs itself more slowly, over days to weeks. Acid suppression with proton pump inhibitors has been shown to reverse the characteristic histologic changes of esophageal reflux including basal layer thickening and dilation of intercellular spaces.77,92
GASTRIC FACTORS
Gastric Acid Secretion
Acid and activated pepsin are the key ingredients of the gastric refluxate producing esophagitis. In animal studies, acid alone causes minimal injury at a pH of less than 3, primarily by protein denaturization. However, acid combined with even small amounts of pepsin disrupts the mucosal barrier, resulting in increased H+ permeability, histologic changes, and hemorrhage.93 Supporting these animal studies, clinical series find that the degree of esophageal injury, from nonerosive GERD to Barrett’s esophagus, parallels the increase in the frequency and duration of acid reflux with a pH of less than 4.94,95 Conversely, perfusing the esophagus of animals with the pepsin solution of pH 4 to 7.5 produces minimal mucosal disruption or change in mucosal permeability.93 These observations are the cornerstone of acid inhibition therapy for the treatment of GERD.
Overall, gastric acid secretion is normal in patients with GERD. For example, Hirschowitz96 compared the gastric acid secretion of 115 patients with esophagitis with more than 500 age-, gender-, and disease-matched controlled subjects without esophagitis. The average fasting basal and maximum secretions of both acid and pepsin were the same in both groups, and esophagitis severity was not related to any of these factors. On the other hand, local distribution of acid rather than total gastric secretion may be more relevant to the pathogenesis of GERD. Data suggest that the gastroesophageal junction may escape the buffering effect of meals, remaining highly acidic (median pH 1.6) compared with the body of the stomach (pH 4.7). This “proximal pocket of acid” extends from the cardia into the distal esophagus and could account for the high prevalence of disease in this location.97 A recent study confirmed these findings, demonstrating a pH less than 4 for 26.5% of the time over 24 hours when measured at the lower esophageal sphincter.98
H. pylori infection, especially with the cagA+ virulent strain, is a “biological antisecretory agent” that lowers gastric acidity, thereby possibly protecting from the development of severe esophagitis and Barrett’s esophagus.99,100 Acid output may be decreased by several mechanisms: (1) the associated severe corpus gastritis, which, over time, progresses to multifocal atrophic gastritis (see Chapter 51); (2) increased gastric alkaline (bicarbonate) secretion, which returns to normal after H. pylori eradication101; and perhaps (3) production of ammonia by the bacteria itself.102 After eradication of H. pylori, the corpus mucosa can regenerate to normal, increasing acid secretion and potentiating reflux in susceptible patients, possibly contributing to the reports of esophagitis after eradication of this organism.103,104 The consequence of returning the stomach to a healthy state is unknown but may be an underlying factor in the increasing prevalence of severe GERD, Barrett’s esophagus, and even adenocarcinoma in Western populations.13,102
Duodenogastric Reflux
Along with acid and pepsin, duodenal contents may be injurious to the esophageal mucosa. Animal studies demonstrate that conjugated bile acids produce their greatest injury in the presence of acid and pepsin, whereas trypsin and the deconjugated bile acids are damaging in a more neutral environment.105 These experiments suggest that duodenogastric reflux into the esophagus predisposes to complications of GERD.106,107 However, the accurate measurement of duodenogastric reflux is difficult. Traditionally, this phenomenon was defined indirectly by measuring the esophageal pH greater than 7 (i.e., “alkaline reflux”).107 However, on the basis of newer technology that accurately measures bilirubin, the most common pigment of bile, independent of pH, we now know that this technique is inaccurate.108 These studies show that acid and bile reflux increase in parallel across the spectrum of GERD, suggesting a synergistic role in the development of esophagitis and its complications.109,110 Additionally, aggressive acid suppression with proton pump inhibitors decreases both acid and duodenogastric reflux by decreasing the volume of gastric contents available to reflux into the esophagus.110
Delayed Gastric Emptying
The importance of delayed gastric emptying in the pathogenesis of GERD is controversial. Early studies found delay in the gastric emptying of solids in up to 50% of reflux patients.111 However, methodologic problems may have invalidated these studies. More recent investigations found only a 6% to 38% incidence of delayed gastric emptying, regardless of the severity of the esophagitis.112,113 Nevertheless, delayed gastric emptying is a major factor contributing GERD in some groups such as diabetic patients with autonomic peripheral neuropathy.
CLINICAL FEATURES
CLASSIC REFLUX SYMPTOMS
Heartburn is the classic symptom of GERD, with patients generally reporting a burning feeling, rising from the stomach or lower chest and radiating toward the neck, throat, and occasionally the back.114 It occurs postprandially, particularly after large meals or after ingesting spicy foods, citrus products, fats, chocolates, and alcohol. The supine position and bending over may exacerbate heartburn. Recent studies have suggested that sleep deprivation as well as psychological or auditory stress may lower the threshold for symptom perception.115–117 Nighttime heartburn may cause sleeping difficulties and impair next-day function.118 When heartburn dominates a patient’s complaints, it has high specificity (89%) but low sensitivity (38%) for GERD as diagnosed by 24-hour esophageal pH testing.119 GERD is usually diagnosed symptomatically by the occurrence of heartburn two or more days a week, although less frequent symptoms do not preclude the disease.120,121 Although an aid to diagnosis, the frequency and severity of heartburn do not predict the degree of esophageal damage.5
Heartburn symptoms can arise from acid reflux, weakly acidic reflux, bile reflux, and mechanical stimulation of the esophagus. The receptor that mediates the sensation of heartburn during acid perfusion has not been identified, although the capsaicin or vanilloid receptor 1 (TRPV1) is a leading candidate. TRPV1 is a cation channel that is expressed by sensory neurons, and its activation by heat, acid pH, or ethanol may trigger burning pain.122–126 Weakly acidic reflux, as detected by combined pH and impedance technology, appears to produce symptoms when there is a large proximal extent reached by the refluxate, large reflux volumes, and prolonged acid-clearance times.127 The mechanism that underlies bile-acid–induced esophageal symptoms is unknown. Bile acids are postulated to induce the release of intracellular mediators via damage to lipid membranes.128 In addition to acid-induced and bile-acid–induced esophageal damage, pepsin can cause direct damage to the esophageal mucosa, leading to dilated intercellular spaces and increased esophageal mucosal permeability.129 Esophageal distention and sustained esophageal contractions are other mechanisms proposed to explain the symptom of heartburn. Balaban and associates used high-frequency endoluminal ultrasound to demonstrate a correlation between spontaneous chest pain or chest pain induced by edrophonium chloride and sustained esophageal longitudinal muscle contractions.130 Esophageal hyperalgesia (lowered pain threshold) and psychological comorbidity have also been postulated to contribute to heartburn symptoms.131
Other common symptoms of GERD are acid regurgitation and dysphagia. The effortless regurgitation of acidic fluid, especially after meals and worsened by stooping or the supine position, is highly suggestive of GERD.119 Among patients with daily regurgitation, LES pressure is usually low; many have associated gastroparesis, and esophagitis is common, making this symptom more difficult to treat medically than classic heartburn. Dysphagia is reported by more than 30% of individuals with GERD.132 It usually occurs in the setting of long-standing heartburn with slowly progressive dysphagia for solids. Weight loss is uncommon, because patients have good appetites. The most common causes are a peptic stricture or Schatzki’s ring, but other etiologies include severe esophageal inflammation alone, peristaltic dysfunction, and esophageal cancer arising from Barrett’s esophagus (see Chapter 44).
Less common symptoms associated with GERD include water brash, odynophagia, burping, hiccups, nausea, and vomiting.133 Water brash is the sudden appearance in the mouth of a slightly sour or salty fluid. It is not regurgitated fluid, but rather secretions from the salivary glands in response to acid reflux.73 Odynophagia may be seen with severe ulcerative esophagitis. However, its presence should raise the suspicion of an alternative cause of esophagitis, especially infections or injury from impacted pills (see Chapter 45).
Some patients with GERD are asymptomatic. This is particularly true in the older adults, perhaps because of decreased acidity of the reflux material in some or decreased pain perception in others.5 Many older adult patients present first with complications of GERD because of long-standing disease with minimal symptoms. For example, up to one third of patients with Barrett’s esophagus are insensitive to acid at the time of presentation.134
EXTRAESOPHAGEAL MANIFESTATIONS
GER may cause a wide spectrum of conditions including noncardiac chest pain, asthma, posterior laryngitis, chronic cough, recurrent pneumonitis, and even dental erosion.135 Some of these patients have classic reflux symptoms, but many are “silent refluxers,” contributing to problems in making the diagnosis. Furthermore, it may be difficult to establish a causal relationship even if GER can be documented by testing (e.g., pH studies) because individuals may simply have two common diseases without a cause-and-effect relationship.
Chest Pain
GER-related chest pain may mimic angina pectoris, having a squeezing or burning quality; being in a substernal location; and radiating to the back, neck, jaws, or arm. It frequently is worse after meals, can awaken the patient from sleep, and may worsen during emotional stress. Heavy exercise, even treadmill testing, may provoke GER.136 Reflux-related chest pain may last for minutes to hours, often resolves spontaneously, and may be eased with antacids. The majority of patients with GERD-induced chest pain have heartburn symptoms.137
Multiple studies since the mid-1990s identify GER, rather than spastic motility disorders, as the most common esophageal cause of noncardiac chest pain.138 The mechanism for GERD-related chest pain is poorly understood and is probably multifactorial, related to H+ ion concentration, volume, and duration of acid reflux; secondary esophageal spasm; and prolonged contractions of the longitudinal muscles.139
Asthma and Other Pulmonary Disorders
The prevalence of GERD in asthmatics is estimated between 34% and 89%, depending on the group of patients studied and how GERD is defined (e.g., symptoms or 24-hour pH monitoring).140 Symptomatic GERD is an important comorbid condition in asthma patients, being associated with greater asthma severity.141 GERD should be considered in asthmatics who present in adulthood, those without an extrinsic (allergic) component, and those not responding to bronchodilators or glucocorticoids.142 Up to 30% of patients with GERD-related asthma have no esophageal complaints. Other pulmonary diseases associated with GERD include aspiration pneumonia, interstitial pulmonary fibrosis, chronic bronchitis, and bronchiectasis. In addition, preliminary data suggest that GER may worsen the course of obstructive sleep apnea in a subset of patients.143
Proposed mechanisms of reflux-induced asthma include aspiration of gastric contents into the lungs with secondary bronchospasm and activation of a vagal reflex from the esophagus to the lungs causing bronchoconstriction. Animal144 and human145 studies report bronchoconstriction after esophageal acidification, but the response is mild and inconsistent. On the other hand, intratracheal infusion of even small amounts of acid induces profound and reproducible bronchospasm in cats.144 The reflux of acid into the trachea as compared with the esophagus alone predictably caused marked changes in peak expiratory flow rates in asthmatic patients.146 Although both mechanisms may trigger reflux-induced asthma, patients with severe asthma probably suffer from intermittent microaspiration.
Ear, Nose, and Throat Diseases
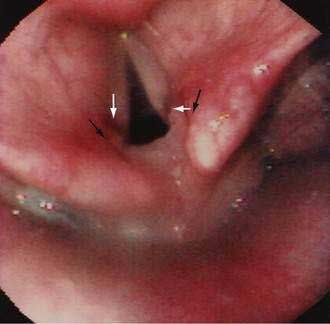
GERD may be associated with a variety of laryngeal symptoms and signs, of which “reflux laryngitis” is the most common.147,148 These patients present with hoarseness, globus sensation, frequent throat clearing, recurrent sore throat, and prolonged voice warm-up. Ear, nose, and throat signs attributed to GERD include posterior laryngitis with edema and redness (Fig. 43-9), vocal cord ulcers and granulomas, leukoplakia, and even carcinoma. These changes are usually limited to the posterior third of the vocal cords and interarytenoid areas, both in proximity to the upper esophageal sphincter. Animal studies find that the combination of acid, pepsin, and conjugated bile acids is very injurious to the larynx.149 Human studies report that proximal esophageal acid exposure, especially while sleeping, is significantly increased in patients with laryngeal symptoms and signs.150
GERD has been postulated to be a leading cause of chronic cough (after sinus problems and asthma).151 GER increases the cough sensitivity reflex (i.e., reduces the cough threshold) in patients with chronic cough.152 However, the importance of this association in humans has not been shown in treatment studies, which, in sum, have not demonstrated a superiority of proton pump inhibitors over placebo.153 Dental erosion, the loss of tooth structure by nonbacterial chemical processes, can be caused by GER in healthy subjects and patients with bulimia.154 Microaspiration of gastric contents is the most likely etiology of these complaints.