CHAPTER 48 Gastric Neuromuscular Function and Neuromuscular Disorders
Gastric neuromuscular function refers to the motility or motor activities of the stomach.
ELECTROPHYSIOLOGIC BASIS OF GASTRIC NEUROMUSCULAR FUNCTION
EXTRACELLULAR SLOW WAVES AND PLATEAU AND ACTION POTENTIALS
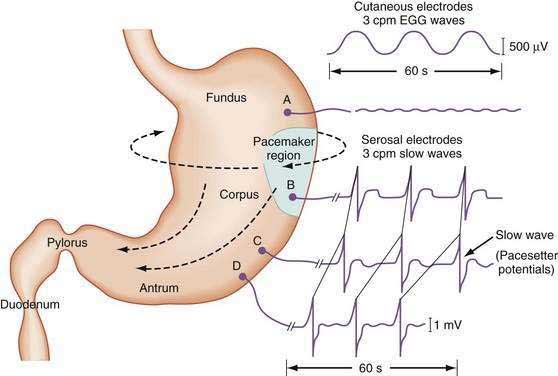
The stomach is a sophisticated complex sphere of smooth muscle organized into circular, longitudinal, and oblique muscle layers. Gastric myoelectrical activity, termed pacesetter potentials or slow waves, regulate, control, and pace gastric smooth muscle contractions1,2 (the term slow waves is used in this chapter). In the normal human stomach the slow waves occur at approximately 3 cycles per minute (cpm) or between 2.5 and 3.7 cpm.3,4 From the pacemaker region on the greater curvature of the stomach, between the fundus and the proximal corpus, slow waves propagate circumferentially and migrate distally toward the pylorus every 20 seconds at a velocity of approximately 14 mm/second in the distal antrum (Fig. 48-1).5,6 The gastric slow waves originate from the interstitial cells of Cajal.7,8
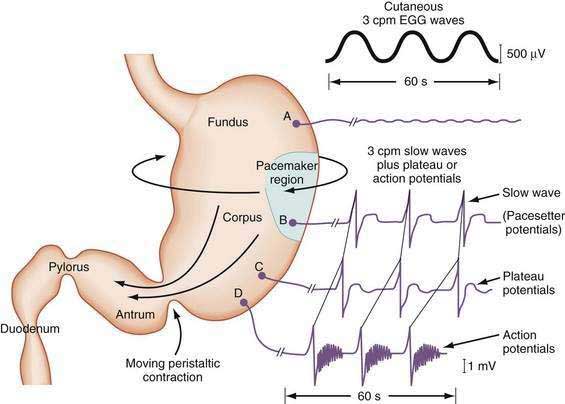
The depolarization upstroke of the slow wave reduces the threshold for circular smooth muscle contraction; and, in the appropriate situation, the amplitude of the circular smooth muscle contraction increases with the onset of the plateau potentials and action potentials.9,10 The aborad propagation of slow waves linked to plateau potentials (with or without action potentials) is the electrophysiologic basis of gastric peristaltic waves (Fig. 48-2). Thus, the slow waves linked with plateau or action potentials propagate through the corpus and antrum and create moving “ring contractions” that resolve in the antrum or at the pylorus in a terminal antral contraction. The pylorus provides an electrical barrier between the 3 cpm slow wave of the distal antrum and the 12 to 13 cpm slow wave of the duodenum.
INTRACELLULAR ELECTRICAL RECORDINGS FROM GASTRIC SMOOTH MUSCLE CELLS
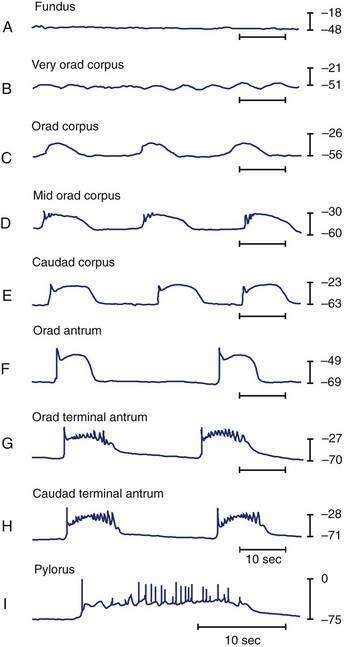
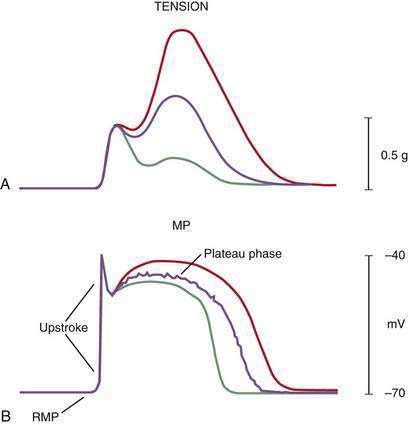
Intracellular recordings from smooth muscle cells from the different regions of the stomach (fundus to the mid-corpus to the terminal antrum) illustrate the electrophysiologic characteristics that distinguish these regions (Fig. 48-3).1 Key features are (1) regional differences in resting membrane potential, which range from −48 to −75 millivolts (mV); (2) regional differences in threshold for contraction that vary from −52 to −40 mV; and (3) the occurrence of plateau potentials with or without spike potentials.1 The fundic smooth muscle cells are unique because their resting membrane potential lies at or above the threshold for contraction (−50 mV), a situation that promotes sustained smooth muscle contraction and ongoing fundic tone. Inhibitory vagal input to the fundus increases during swallowing and results in decreasing muscle tone associated with “receptive relaxation” and the accommodation of swallowed foodstuffs.11,12 Fundic muscle tone decreases in proportion to the intensity and duration of the inhibitory neural discharge.
In contrast to the fundus, intracellular recordings from the corpus indicate a lower resting membrane potential of −60 mV. The rapid upstroke depolarization in these cells is followed by a plateau potential that slowly returns to the baseline resting electrical potential (see Fig. 48-3F). The plateau potentials are associated with circular muscle contraction activity in the corpus and antrum.1 The plateau potential may be accompanied by action potentials in the corpus and antrum (see Fig. 48-3G and H). Extrinsic stimuli such as release of acetylcholine or stretch of the stomach wall increases the amplitude and duration of the plateau potential and the occurrence of action potentials, resulting in contractions of varying force, as seen in the muscle of the terminal antrum. Depending on the excitatory neural stimuli and the amplitude of plateau potentials and the number of action potentials, peristaltic contraction waves of the circular muscle layer vary from very-low-amplitude contractions to high-amplitude lumen-occluding contractions. At the pylorus, the plateau potentials have long durations and superimposed action potentials that result in closure of the pyloric sphincter in conjunction with the terminal antral contraction (see Fig. 48-3H and I).1
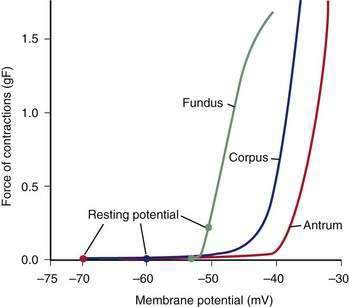
The membrane potential and the force of smooth muscle contraction also distinguish the fundus, corpus, and antrum (Fig. 48-4). The resting membrane potential of the fundus is approximately −50 mV and produces the sustained contraction and the resting tone of the fundus.1 This fundic tone ensures a sensitive response to excitatory or inhibitory stimuli for relaxation or contraction of the fundus. Receptive relaxation during ingestion of food is accomplished by these electrophysiologic attributes of smooth muscle in the fundus. In contrast, the resting membrane potentials of the corpus and antrum are −60 to −70 mV, respectively. In the presence of plateau potentials or action potentials the membrane potential reaches −45 mV or less and smooth muscle contraction occurs. If the plateau potentials have higher amplitude, then contractions of larger amplitude or force occur. When the plateau potential and action potentials are linked to the propagating slow waves in the antrum, then the moving ring contractions of the gastric peristaltic “waves” are formed (see Fig. 48-2).
INTERSTITIAL CELLS OF CAJAL
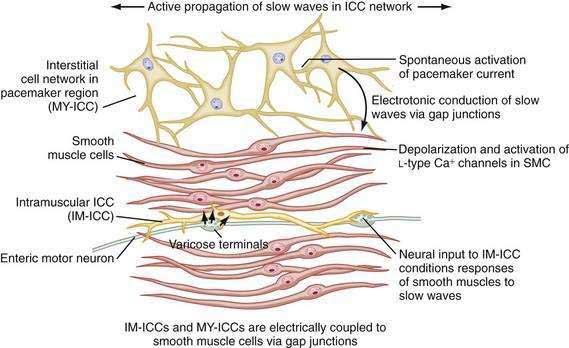
ICCs are the “pacemaker cells” for the smooth muscle apparatus of the gastrointestinal tract.7,8,13,14 ICCs originate from c-Kit–positive mesenchymal cell precursors.15 ICCs in the stomach are located in submuscular, intramuscular, myenteric, and subserosal layers of the gastric wall.16,17 Figure 48-5 shows the anatomic relationships between the ICCs in the myenteric plexus (MY-ICCs), the intramuscular ICCs (IM-ICCs), the enteric neurons, and the circular smooth muscle cells. MY-ICCs are located between the circular and longitudinal muscle layers of the stomach and are the ICCs responsible for the generation of the slow waves (see Fig. 48-5). These ICCs spontaneously generate slow waves that are conducted into adjacent smooth muscle cells and cause depolarization and contraction of the smooth muscle by activating voltage-dependent, dihydropyridine-sensitive (l-type) calcium channels (see Fig. 48-5).18–20 Increased amplitude of the plateau potential correlates with increased amplitude of smooth muscle contraction (Fig. 48-6). The slow waves propagate circumferentially and distally through the ICC network via gap junctions and entrain more distal ICCs with slower intrinsic frequencies to the higher slow wave frequency, the pacemaker frequency.
ICCs are also located within the layers of the circular smooth muscle (IM-ICCs), where they integrate and coordinate the spread of the slow wave and the smooth muscle contraction initiated by the MY-ICCs.10 Slow waves are not regenerated in the smooth muscle cells because the ion channels needed to generate and propagate slow waves are not expressed by gastric smooth muscle.
The ICCs have innate rhythmicity that is based on their unique metabolism and fluxes in intracellular and extracellular calcium.19,21 The most active area of depolarization and repolarization of the ICCs is in the pacemaker area of the stomach located between the fundus and the proximal corpus (see Fig. 48-1). The depolarization and repolarization of the ICCs is regenerated and propagated through the network of ICCs in a migrating wave front that moves from the pacemaker region on the greater curve through the corpus and antrum to the pylorus, as shown in Figures 48-1 and 48-2, proscribing the pathway of gastric peristaltic contractions. Excitatory inputs (e.g., cholinergic stimuli, stretch) to the MY-ICC results in opening of calcium channels and depolarization of the smooth muscle cells with IM-ICC activation to coordinate the contractions of the circular muscle cells in time and space. Thus, the ICC networks provide the control of frequency and propagation velocity for the circular muscle contractions that comprise gastric peristalsis waves.
The fundus of the stomach lacks slow waves (see Figs. 48-1 and 48-3A). The IM-ICCs in the fundus have a role in mechanoreception and act as sensory cells with interconnections to the vagal afferent neurons that innervate the fundus.22,23 Fundic IM-ICCs are also innervated by inhibitory vagal neurons that regulate tone in the fundus.23 Thus, the ICCs also participate in the relaxation of fundic tone that occurs during accommodation.24
Loss of ICCs in the antrum is associated with gastroparesis in patients with diabetes mellitus.25 Patients with diabetic gastroparesis and severe loss of ICCs have more gastric electrical dysrhythmias (tachygastria), more upper gastrointestinal symptoms, and poorer response to gastric electrical stimulation compared with patients with normal numbers of ICCs.26 Electrical dysrhythmias and gastroparesis were recorded in rodents with experimental diabetes.27 Interruption of ICC pathways from nondiabetic mechanisms also results in gastric dysrhythmias and ectopic pacemakers that are similar to gastric dysrhythmias found in patients with diabetes.28,29
NERVOUS SYSTEM INNERVATION
As reviewed earlier in Chapter 47, neurons of the ENS populate the stomach wall from the fundus to the pylorus.30 These neurons are located in the myenteric plexuses between the circular muscle and the longitudinal muscle layers (see Fig. 48-5). Neurons of the ENS are also located in submucosal and subserosal plexuses. The ENS provides local reflex circuits within the gastric wall: (a) sensory afferent neurons located in the mucosa linked to (b) interneurons in the myenteric plexus that are linked to (c) efferent neurons that innervate the smooth muscle and glands to perform the gastric secretomuscular functions.31,32 Release of excitatory neurotransmitters such as acetylcholine and substance P stimulates smooth muscle contractions, whereas inhibitory neurotransmitters such as nitric oxide and vasoactive intestinal polypeptide inhibit contractions. These enteric neural circuits within the gastric wall are programmed to modulate peristaltic contractions (in conjunction with ICC activity described earlier) by sequential inhibition of the distal smooth muscle segment and contraction at the immediate proximal segment of the stomach wall.33,34 Serotonin in the bowel wall has a primary role in initiating and controlling peristaltic events.32
Neurons of the ENS are located in proximity to the MY-ICCs and IM-ICCs (see Fig. 48-5).35 The ENS neurons provide additional control and modulation of contraction and relaxation of the gastric smooth muscle via cholinergic excitation and nitrergic inhibitory neurotransmission. Neurons of the ENS form gap junctions with MY-ICCs and IM-ICCs and provide an additional layer of neural control that integrates slow wave activity and smooth muscle activity. Thus, postganglionic excitatory and inhibitory neurons innervate MY-ICCs to modulate gastric neuromuscular contraction and relaxation and provide chronotropic effects on the slow waves.
The PNS and SNS modulate gastric neuromuscular activity. The vagus nerve provides the PNS input for the stomach, although approximately 80% of vagal fibers are afferent neurons. The afferent neurons are responsive to moment-to-moment contraction and relaxation (tone) of the stomach wall.36 Efferent activity of the vagus nerve increases the release of acetylcholine, which increases the amplitude of gastric contractions and stimulates secretion of gastric acid and pepsin. The SNS innervates gastric smooth muscle with neurons that travel with the splanchnic vasculature. SNS activity generally elicits inhibitory action on the smooth muscle via effects on the myenteric neurons of the ENS.37
GASTRIC NEUROMUSCULAR ACTIVITY DURING FASTING
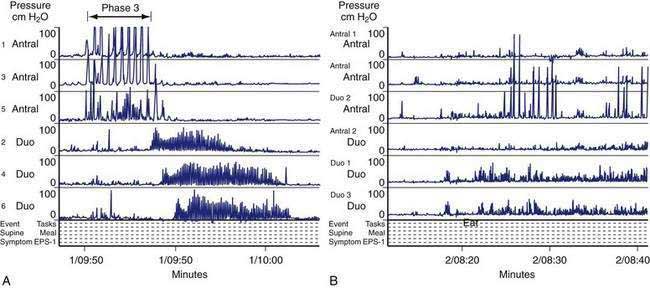
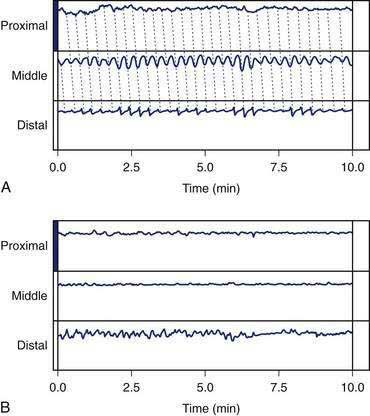
In the fasting state, electrical and contractile events of the corpus or antrum occur in a highly regular pattern termed the migrating myoelectrical (or “motor”) complex, or MMC.38,39 The three phases of the MMC, as described by changes in intraluminal contractions, recur approximately every 90 to 120 minutes. Phase 1 is a period of quiescence wherein little or no contractile activity is recorded. Phase 1 is followed by phase 2 during which random, irregular contractions occur. Phase 3 of the MMC is a burst of regular, high-amplitude phasic contractions that last from 5 to 10 minutes (Fig. 48-7A). Phase 3 contractions are also termed the “activity front.” The activity front migrates from the antrum to the ileum, a journey of 90 to 120 minutes’ duration. The three phases of the MMC occur regularly in the small intestine, whereas approximately 50% of the phase 3 activity fronts originate in the stomach and then migrate through the small intestine.40 The MMCs that originate in the stomach or duodenum travel through the small intestine and terminate in the distal ileum. If fasting continues, then another phase 3 activity front reappears in the antrum or duodenum at the 90- to 120-minute interval. The high-amplitude, three-per-minute contractions of phase 3 that develop in the distal antrum empty nondigestible, fibrous foodstuffs that remain in the stomach after a meal.
Cyclic contractile activity associated with the onset of phase 3 also has been identified in the lower esophageal sphincter, the sphincter of Oddi, and the gallbladder. The phase 3 contractions correlate with rapid eye movement (REM) sleep and are related to a larger system of biological clocks.38,41 MMCs develop after vagotomy, indicating that nonvagal mechanisms initiate and sustain MMC neuromuscular activity. Motilin is released during the intense phase 3 contractions that occur in the proximal duodenum.42 Motilin appears to be important for phase 3 activity of the MMC because administration of a motilin-neutralizing antibody abolishes the phase 3 contractions.
GASTRIC NEUROMUSCULAR ACTIVITY AFTER A MEAL
Gastric Neuromuscular Response to the Ingestion of Solid Foods
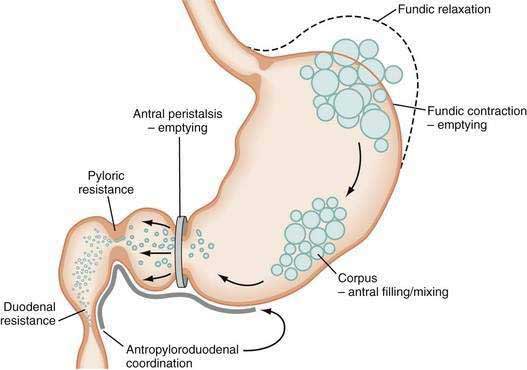
The neuromuscular work of the stomach in mixing, milling, and emptying food depends upon the physical characteristics, volume, and the fat, protein, and carbohydrate content of the ingested food. For example, almost 240 minutes of neuromuscular work is required to empty approximately 95% of a 255-kcal low-fat, egg substitute sandwich.43 In contrast, 35 minutes of gastric neuromuscular work is required to empty almost 70% of a 20-kcal 500-mL soup broth meal that was consumed in four minutes.44 Each meal requires its own specific time to be emptied, an emptying time achieved by the distinct gastric neuromuscular “work” elicited by the specific food. Figure 48-8 illustrates gastric neuromuscular activity required to receive, mix, and empty a solid meal. The spectrum of gastric work extends from fundic relaxation to gastric peristalsis to antropyloroduodenal coordination, the work that is needed to produce chyme and empty it into the duodenum. Ingestion of food abolishes the fasted state as regular three per minute gastric peristalsis begins in the corpus and antrum to mix the food; and in the fed state, a pattern of continuous small bowel contractions with short runs of peristalsis over distances of 2 to 4 cm optimize digestion and absorption of nutrients (see Fig. 48-7B).
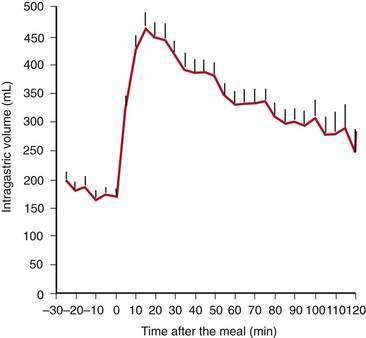
Solid food delivered from the esophagus into the fundus is associated with receptive relaxation of the fundus, the “work” of fundic muscle relaxation. As the fundic smooth muscle relaxes, larger amounts of solid or liquid food are accommodated in the fundus and proximal corpus with little or no increase in intraluminal pressure. Liquids, in contrast, are immediately distributed throughout the antrum and corpus (emptying of liquids is discussed in the next section). Relaxation of the fundus occurs before the work of trituration in the corpus-antrum and is a vagal nerve–mediated event that requires nitric oxide.45,46 Figure 48-9 shows an example of the changes in intragastric volume during relaxation of the fundus and proximal corpus in response to a caloric meal.47 Relaxation of the fundus and the stimulation of mechanoreceptors (stretch), mediated through IM-ICCs in the fundic wall, activate vagal afferent neurons and vagovagal reflexes. These reflexes involve the nucleus of the tractus solitarius and efferent neurons from the dorsal motor nucleus of the vagus. Vagal excitatory neurons are inhibited and the vagal inhibitory neural transmitters nitric oxide and vasoactive intestinal peptide (VIP) are released to accomplish receptive relaxation.
Other factors influence the muscle tone of the fundus. Antral distention, duodenal distention, duodenal acidification,48 intraluminal perfusion of the duodenum with lipid or protein, and colonic distention all decrease fundic tone through various reflexes. The gastric reflex is mediated through an arc initiated by capsaicin-sensitive afferent vagal nerves and is mediated by 5-hydroxytryptamine-3 (5-HT3), gastrin-releasing peptide (GRP) and CCKA receptors.49
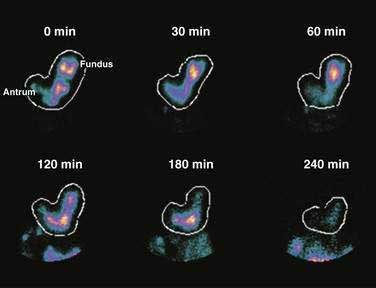
Solid foods labeled with technetium are accommodated initially in the fundus and proximal corpus, and by obtaining frequent scintigraphic images, the distribution of the labeled solid meal can be followed over four hours using scintigraphic methods.43 Figure 48-10 shows that immediately after ingestion of this solid meal, the food is accommodated and the majority of the meal is retained in the fundus and proximal corpus. Subsequently, contractions of the fundus press portions of the food into the corpus and antrum for trituration. This early postprandial period of accommodation and trituration, that occurs before gastric emptying of the nutrients, is termed the lag phase. The lag phase may last from 45 to 60 minutes for solid foods, but the duration of the lag depends on the components of the meal, the thoroughness of chewing the food, and the time required to ingest the meal. For the 255-kcal egg substitute test meal that is ingested in a 10-minute period, the lag phase is 30 to 45 minutes.
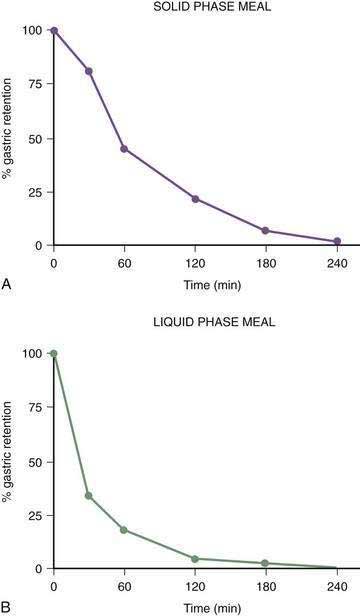
Once portions of the meal have been triturated into 1- to 2-mm particles suspended in gastric juice, the linear phase of gastric emptying of the chyme begins. Recurrent gastric peristaltic waves mix saliva, acid, and pepsin with the chewed food and then mill the food to produce chyme. The normal peristaltic waves occur every 20 seconds, generated by 3 cpm slow waves linked to plateau and action potentials. In healthy subjects approximately 60% of the egg substitute meal is emptied in two hours and more than 95% is emptied at four hours (Fig. 48-11).43
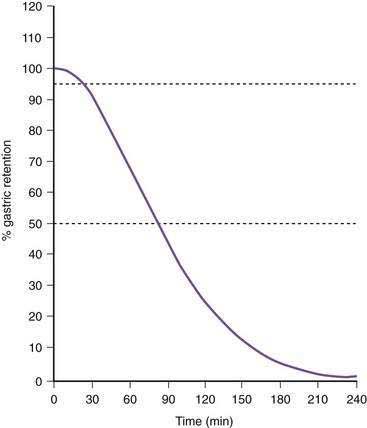
Figure 48-11. Solid phase gastric emptying curve for 123 subjects after ingestion of a 255-kcal substitute egg meal, the same meal shown in Figure 48-10. Note that only approximately 15% of the eggs are emptied in the first 45 minutes, the lag phase of gastric emptying of this meal. At 90 minutes approximately 50% of the meal has been emptied and 50% is retained. By 240 minutes more than 95% of the meal has been emptied.
(Modified from Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am J Gastroenterol 2000; 95:1456-62.)
During the linear phase of gastric emptying, each peristaltic wave empties from 3 to 4 mL of chyme into the duodenum.50,51 Movement of chyme into the duodenum is usually, but not always, pulsatile due to the systole-like effect of antral peristaltic waves.51 The volume of chyme delivered into the duodenum by each peristaltic wave is modulated by the configuration of the peristaltic wave (e.g., depth of contraction, length of the peristaltic wave) pressure within the stomach, and resistance to flow provided by the pyloric sphincter and duodenal contractions.52,53 The gastric peristaltic wave delivers a larger stroke volume when the pylorus and the duodenum are relaxed to receive the aliquot of chyme, but the overall rate of calories delivered each minute to the duodenum is consistent at approximately 3 to 4 kcal/min.54
As time elapses after ingestion of the meal, the chewed food is continually redistributed from the fundus to the antrum for trituration (see Figs. 48-8 and 48-10). Some gastric peristaltic waves end at various points in the antrum and others end with a terminal antral contraction associated with closure of the pylorus that prevents the emptying of larger food particles or indigestible solids. These terminal antral and pyloric contractions result in retention of the solid particles in the corpus and antrum. In this manner, solid food particles that require further trituration are retained and subjected further to the milling effects of the recurrent peristaltic waves (see Figs. 48-7B and 48-8).
The intragastric pressure and gastric intraluminal pH values recorded after a healthy subject ingested an egg substitute meal are shown in Figure 48-12. During the first 2 hours after the meal the amplitude of intraluminal contractions varies from 10 to 40 mm Hg. Approximately three and a half hours after the solid meal was ingested, high amplitude contractions (>65 mm Hg) occur just before the pH increases from 1 to 6 as the motility/pH capsule is emptied from the acidic antrum into the alkaline environment of the duodenum. After the digestible components of the meal are emptied, strong antral contractions (phase 3–like contractions) empty the capsule from the stomach into the duodenum.55 Thus, fibrous and indigestible materials are emptied by high-amplitude, antral contractions, whereas the digestible nutrients in the chyme are emptied earlier by the lower-amplitude peristaltic waves during the linear phase of emptying.56
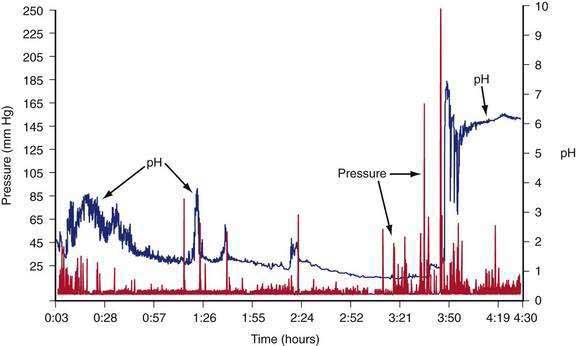
Figure 48-12. Gastric contractions and intraluminal gastric pH recordings during the emptying of a 255-kcal egg substitute meal, the same meal shown in Figure 48-10, recorded with an ambulatory capsule pH and motility device. pH is shown on the right vertical axis and pressure in mm Hg is shown on the left vertical axis. The pH increases to approximately 3 for the first 45 minutes as gastric acid is buffered by the meal. The pH then gradually decreases to 1 and remains near 1 at about 3 hours after ingestion of the meal. Stomach contractions are generally of low amplitude, less than 10 mm Hg after ingestion of the meal. At approximately 3 hours and 40 minutes after the meal, the recorded pH increases abruptly to 7 and then decreases and remains stable at around 6. Prior to the abrupt increase in pH, there is a series of clustered, high-amplitude antral contractions (pressure). These antral contractions empty the capsule from the antrum (pH 1) into the duodenum, where the pH is 6 or more. The contractions that occurred during the 3 hours and 50 minutes required to empty the meal document the neuromuscular work required to triturate and empty this meal in a healthy subject.
Gastric Neuromuscular Response to the Ingestion of Liquids
The gastric neuromuscular activity required to mix and empty liquids from the stomach is distinctly different compared with the emptying of solid foods.55–59 Figure 48-13 shows three-dimensional (3-D) ultrasound images of the stomach in a healthy subject during the fasting state and 10 minutes after the subject ingested 500 mL of soup.58 The intragastric volume was approximately 40 mL during fasting and increased almost nine-fold to 350 mL 10 minutes after ingestion of the meal, indicating the remarkable relaxation of the smooth muscle of the antrum and corpus (in addition to the fundic relaxation) that was required to accommodate this liquid volume. (In contrast, solid meals are initially accommodated and retained primarily in the fundus and proximal stomach, as shown in Figs. 48-9 and 48-10.) Once accommodated, nutrient liquids are emptied into the duodenum in a controlled but more rapid rate compared with solid foods, which require trituration. Noncaloric liquid meals empty without the lag phase in a curve described as monoexponential emptying (Fig. 48-14).60 Caloric-dense liquids on the other hand are retained for longer periods in the antrum and are emptied slower than noncaloric liquids. Liquids are emptied from the stomach by a combination of (a) pressure gradients between the stomach and the duodenum that produce flow of liquid into the duodenum; (b) antral peristaltic contractions that produce a pulsatile pattern of emptying of liquids from the antrum into the duodenum; and (c) duodenogastric reflux events that modify gastric emptying results.51,52 From a gastric myoelectrical activity viewpoint, ingestion of water until the point of fullness induces a brief “frequency dip” followed by normal 3 cpm activity on the electrogastrogram (EGG) (Fig. 48-15).61 The rate of gastric emptying of liquids is influenced by the volume, nutrient content, viscosity, and osmolarity of the ingested liquid.53,54,59,62 These factors affect the neuromuscular activity of the stomach, which ultimately produces the rate of emptying. These factors are discussed below.
REGULATION OF GASTRIC NEUROMUSCULAR ACTIVITY AFTER A MEAL
Gastric emptying rates are regulated to achieve a consistent, regular presentation of chyme to the duodenum in order to optimize secretion of pancreatic enzymes and bile appropriate for digestion of the contents of the chyme. Various gastric emptying rates are achieved by variations in the neuromuscular armamentarium of the stomach: fundic relaxation and contraction; the characteristics of gastric peristaltic contractions; temporary suspension of 3 cpm slow waves and the onset of gastric dysrhythmias; the coordination of antropyloroduodenal contractions and duodenal contractions; or resistances that promote duodenogastric reflux. The attributes of a specific meal stimulate the appropriate gastric neuromuscular responses that affect the rate of gastric emptying. Table 48-1 lists gastric neuromuscular factors, meal-related factors, and other factors that modulate the rate of gastric emptying. The rate of gastric emptying is decreased by the temporary occurrence of gastric dysrhythmias, modulation of the amplitude and the propagation distances of antral contractions, enhanced contractions of the pylorus, and reduced antropyloroduodenal coordination.
Table 48-1 Factors That Modulate the Gastric Emptying Rate
| FACTORS | EFFECT ON RATE OF GASTRIC EMPTYING (SYMPTOMS) |
|---|---|
| Gastric Neuromuscular | |
| Meal-Related Factors | |
| Small Intestinal Factors | |
| Colonic Factors | |
| Constipation, IBS | Delay |
| Other Factors | |
CHO, carbohydrate; IBS, irritable bowel syndrome.
Meal-related factors that affect gastric emptying include the digestible components of the solids and liquids, fat content (nutrient density), viscosity, acid content, volume, and indigestible foodstuffs. For example, foods with high fat content empty slower than foods with high protein or carbohydrate content. Triglycerides are mixed with gastric lipase during the initial intragastric phases of digestion (Chapter 49) and are broken down to fatty acids and mono- or diglycerides before emptying into the duodenum.63 The duodenum is exquisitely sensitive to diet-derived fatty acids. Longer chain fatty acids (longer than C12) exposed to the mucosa of the duodenum result in release of CCK. CCK relaxes fundic tone, decreases antral contraction, and increases pyloric tone, all of which result in delay in gastric emptying. In contrast, short- and medium-chain fatty acids (shorter than C12) do not have these neuromuscular effects on gastric emptying rates.54,64 CCK released from the duodenum also activates CCKA receptors on the vagal afferent neurons with synapses in the nucleus tractus solitarius.65 Neurons from the nucleus tractus solitarius ascend to the periventricular nucleus that participate in mechanisms of satiation, and descending vagal efferent neurons from the dorsal motor nucleus of the vagus inhibit gastric emptying and maintain fundic relaxation. The sensitivity of the duodenal mucosa to fat and other nutrients led to the concept of duodenal tasting and duodenal brake, sensorimotor events that modulate gastric emptying of nutrients.66–68
Monosaccharides in the duodenum stimulate the release of incretins such as glucagon-like polypeptide-1 (GLP-1), which promotes insulin secretion to match increasing postprandial blood glucose levels and decreases antral contractions.69–71 In order to harmonize the relationships between glucose absorption, glycemia, and insulin secretion, the gastric emptying of carbohydrates is highly regulated.72 Hasler and colleagues showed that hyperglycemia decreases antral contractions and increases gastric dysrhythmias, a “physiologic” gastric dysrhythmia that decreases the rate of gastric emptying (Fig. 48-16).6,73 Glucagon infusions also induce bradygastrias.74 Hyperglycemia increases fundic compliance and decreases sensations related to fundic distention.75 Blood glucose levels greater than 220 mg/dL result in decreased antral contractions, decreased gastric emptying, and induced gastric dysrhythmias,6,73 all of which are gastric neuromuscular activities that reduce gastric emptying and reduce further exposure of the duodenum to nutrients. Hypoglycemia on the other hand increases gastric contractility and emptying.76
The interaction between nutrients in the lumen and the regulation of the rate of gastric emptying continues in the later postprandial period as digestion and absorption of nutrients occur throughout the small intestine. For example, if diet-derived fatty acids or carbohydrates reach the lumen of the ileum, the so-called ileal break is activated and gastric emptying is delayed. Infusion of nutrients into the lumen of the ileum delays gastric emptying,77 an enterogastric reflex mediated in part by peptide YY, CCK, and GLP-1 (see Chapter 1).61,68,77
Regulation of stomach emptying also is achieved by vagus nerve and splanchnic nerve activity that modulates the neuromuscular activities of the stomach described earlier. Vagal afferent nerves “monitor” neuromuscular function in the stomach moment by moment, and interactions between afferent vagal nerve activity and the nucleus tractus solitarius and synapses with the efferent vagal nerve output from the dorsal motor nucleus produce an ongoing interaction of CNS excitatory and inhibitory effects on the stomach. Gastric emptying is delayed during stress. Corticotropin-releasing factor (CRF) plays a role in the mediation of stress and inhibits gastric emptying through central dopamine1 and 2 and vasopressin (AVP) pathways in the periventricular nucleus.78 Other factors that affect the rate of gastric emptying not already mentioned include rectocolonic distention, nausea and vomiting of pregnancy, and vection-induced motion sickness.79 Stimulation of various areas in the CNS affects gastric neuromuscular function. Illusory self-motion (vection) induces antral hypomotility, tachygastria, and decreased gastric emptying.79,80 A series of studies using the experience of illusory self-motion, a unique CNS sensory stimulation, showed that the onset of nausea was associated with tachygastria and increased levels of plasma vasopressin.80,81
Gender affects the gastric emptying rate of a standard meal. Gastric emptying is significantly slower in healthy women compared with men.43,82,83 Gender differences in gastric emptying rates may be related to fluctuations in sex hormones, but phases of the menstrual cycle (variations in estradiol and progesterone concentrations) have not shown consistent relationships with emptying measurements.84 The rate of gastric emptying increases as body mass index rises, a relationship that may be relevant to the onset and maintenance of obesity.
GASTRIC SENSORY ACTIVITIES
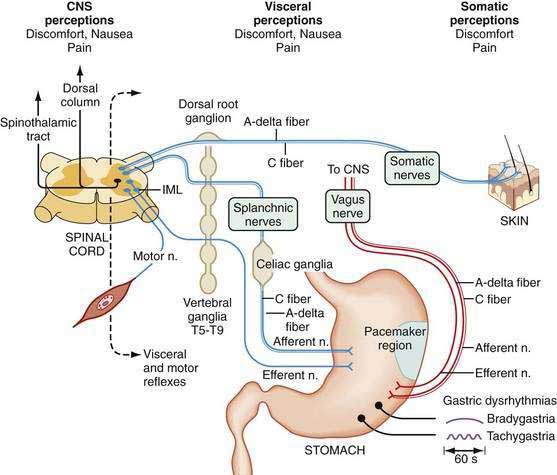
Free nerve endings in the stomach act as polymodal sensory receptors that respond to light touch or pressure, acid, and other chemical stimuli. Afferent neurons within the stomach are termed intrinsic primary afferent neurons, or IPANs.85 Cell bodies of IPANs reside in the submucosal or the myenteric plexus areas of the stomach wall. IPANs may be activated by serotonin release from local enterochromaffin cells.32,86 The afferent information in the IPANs is used in local reflexes and provides input to vagal and splanchnic afferent neurons for vagovagal and spinal reflexes, respectively, to subserve transmission of visceral sensory information to CNS centers. Vagal afferent neurons whose cell bodies reside in the nodose ganglia connect with the nucleus of the tractus solitarius and second-order neurons connect with higher center of the hypothalamus, and some inputs reach the cortex, where they are consciously perceived as visceral sensations (stomach emptiness or fullness) or symptoms such as nausea or abdominal pain (Fig. 48-17).
From the SNS, splanchnic or spinal primary afferent neurons in the gastric wall mediate pain sensations. Cell bodies of these neurons lie in the dorsal horn of the spinal cord with second-order neurons that ascend via the spinothalamic and spinoreticular tracks in the dorsal columns. Sensory neurons are thin, myelinated A-delta or unmyelinated C fibers. Spinal afferents include a population of unmyelinated C fibers. Capsaicin-sensitive unmyelinated fibers contain neuropeptides such as CGRP, VIP, somatostatin, substance P, and neurokinin A. These fibers are considered to be the primary route of transmission for various pain stimuli from the gut to the CNS. These nerve fibers may respond to inflammatory mediators that also awaken “silent” nociceptive fibers.86
In addition to interacting with IPANs, vagal afferent axons have multiple connections with the enteric neurons and innervate the circular muscle fiber bundles via connections with ICCs.87 Vagal afferent neurons are also sensitive to chemostimuli via mucosal neurons and mechanosensitive neurons and ICCs in the muscle layers. CCK receptors on vagal afferent neurons are primarily activated by physiologic mechanical and chemical stimuli from the stomach during fasting and fed conditions. These vagal afferents mediate the sensory response to intraluminal acid and fat. Acid may have a direct action on the nerve endings themselves.88
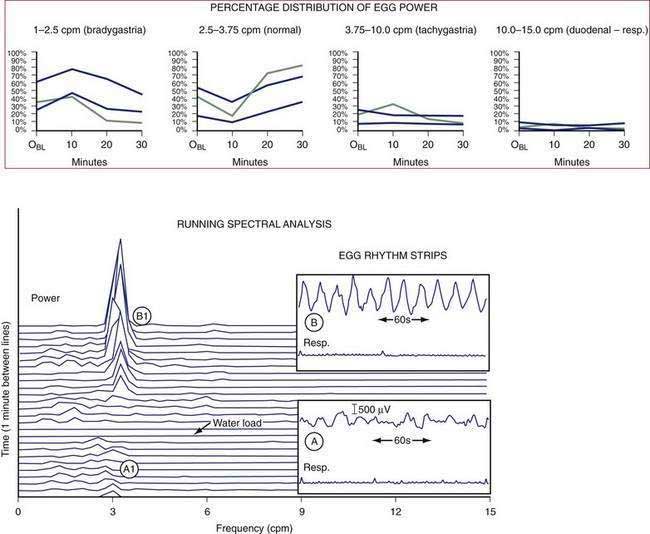
Nausea is a common sensation that is often attributable to stomach dysfunction. During the illusion of self-motion, gastric dysrhythmias develop as healthy individuals report nausea.89 Plasma vasopressin levels increase in the subjects who develop nausea, but do not increase in those who experience no nausea.90 This brain-gut, gut-brain interaction during illusory self-motion illustrates the temporal relationships between the onset of gastric dysrhythmias in the periphery and acute, severe nausea experience of the subject. On the other hand, mechanical or physical distention of the antrum, but not the fundus, using a balloon induces nausea sensations and gastric dysrhythmias in healthy individuals.91 These studies show that gastric dysrhythmias originate in the antrum in humans and that stretch of the antral wall is another mechanism that elicits gastric dysrhythmias and nausea sensations from the stomach. Distention of the gastric antrum and corpus by the water-load test (rather than a balloon) also elicits the gastric dysrhythmias and nausea in susceptible individuals.61
THE STOMACH AND THE REGULATION OF FOOD INTAKE, HUNGER, AND SATIETY
The volume of food ingested suppresses hunger and stimulates the sense of fullness more than the calorie content of the meal.92–94 Infusion of nutrients into the stomach induces a greater intensity of fullness or satiety compared with infusion of the same nutrients into the duodenum.67 The suppression of hunger is greater when nutrients are taken by mouth, indicating that CNS, oropharyngeal, and gastric neuromuscular factors are integrated to produce the comforts of normal postprandial stomach fullness.95
Healthy individuals usually eat until they are reasonably full. The physiologic attributes of fullness are not completely known, but the physical stretch on the stomach walls induced by the volume of food ingested and the gastric juice secreted are responsible, in part, for the sense of postprandial fullness.93,96 Subjects experience a dramatic change from the sensation of stomach emptiness at baseline to the sensation of stomach fullness after ingesting water over a five-minute period. The average volume of water ingested to achieve fullness is 600 mL of water; in contrast, patients with functional dyspepsia ingest, on the average, 350 mL of water on average and feel full, indicating a disturbance in stomach wall relaxation and/or wall tension.61 Similarly, fullness and satiety can be achieved by ingesting a nutrient drink until achieving maximum tolerated satiety.97 The presence of acid or nutrients in the duodenum or an elevated blood glucose level decreases the stomach wall tension.98,99
The physiologic mechanisms of hunger and satiety (and stomach emptiness and fullness) are under intense investigation. In the fasting state plasma motilin levels increase during the phase 3 of the MMC, but correlations between the sensation of hunger and increases of motilin or onset of phase 3 have not been described. As discussed in other chapters, ghrelin is a 28 amino acid peptide secreted from endocrine cells of the oxyntic glands in the gastric fundus.100 Ghrelin levels increase in the plasma during fasting (hunger) and stimulate food intake, probably acting via vagal afferent nerves.101 Orexins or appetite-stimulating peptides are synthesized by neurons in the lateral hypothalamus, promote food intake, and stimulate gastric contractility (in the rat) by actions on the dorsal motor nucleus of the vagus with projections to the gastric fundus and corpus.102 After ingestion of food, ghrelin levels decrease103 and are profoundly suppressed after gastric bypass surgery.104 Ghrelin also has promotility effects on the stomach and is being evaluated for the treatment of gastroparesis.105,106
Other hormones are candidates for important roles in the sensation of fullness or satiety, and these hormones are released after the ingestion of meals. CCK is released from the duodenal mucosa exposed to fatty acids as described previously. CCK receptors participate in fullness and nausea sensations elicited by intraduodenal lipid and gastric distention.107,108 Leptin is synthesized in the stomach and released after food ingestion; circulating leptin reduces food intake via CNS regulation of the arcuate nucleus.71,109 GLP-1 enhances fullness after a standard meal, reduces antral motility, and increases gastric volume.71,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree