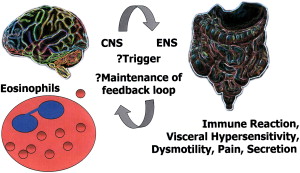
The eosinophil–mast cell–neural pathway may be important in the pathophysiology of functional gastrointestinal disorders characterized by unexplained abdominal pain, disordered defecation, or meal-related discomfort. There is evidence that duodenal eosinophils are increased in functional dyspepsia, whereas mast cells are increased in the lower gut in irritable bowel syndrome, directly supporting a role for a hypersensitivity-type reaction in these disorders. The trigger may be a pathogen, food, or other allergen in the gut mucosa. This trigger may evoke eosinophils, mast cells, and other components to cascade to up-regulate serotonin release, with modulation of the enteric and central nervous systems, creating a vicious cycle. If correct, this theory suggests treatment should specifically target the eosinophil–mast cell pathway.
Functional Gastrointestinal Disorders
The Rome III classification of the functional gastrointestinal disorders (FGIDs) defines 28 adult and 17 pediatric symptom-based gut conditions that have no established pathology . These disorders place a considerable burden on individual patients, because quality of life is poorer in patients who have FGIDs compared with health and is comparable to organic GI disease . There is also a major societal burden because of high direct and indirect costs . Currently, diagnosis is based on symptoms or by exclusion, because there is no objective diagnostic test for these disorders; by definition it is necessary to exclude other diseases, specifically evidence of an inflammatory, anatomic, metabolic, or neoplastic process that explains the patient’s symptoms .
Abdominal pain, disordered defecation, and meal-related discomfort are common symptomatic themes for a range of FGIDs. Treatment remains empirically based on relieving symptoms rather than treating abnormal pathophysiology. The current accepted conceptual model of disease reflects an interaction between psychosocial factors, including stress and anxiety, that impinge on gut physiology by way of the brain–gut axis to produce visceral hypersensitivity or dysmotility. These signals interact by way of the gut enteric nervous system (ENS) and track back to the central nervous system (CNS) . It is possible that this viscious cycle originates in gut mucosa, where an insult triggers a feedback loop, although how often this is the case remains uncertain. Key barriers in attempting to define an organic pathology for FGIDs include a lack of understanding of how symptoms are triggered and how any feedback loop is maintained.
The pathophysiology of the FGIDs has been studied extensively to try to identify a common link for symptom expression. It is currently assumed that there is not a simple or universal pathway to these differing outcomes, but a complex interaction of genetic predisposition, visceral hypersensitivity, and psychosocial distress in a primed central and enteric nervous system .
Eosinophils and Functional Gastrointestinal Disorders
The gut is an active immune organ that is the first line of defense for many insults, such as allergens in food, drugs, viruses, bacteria, and parasites. The authors surmise that altered immune function, by way of infection or hypersensitivity on a background of genetic predisposition and environment, triggers symptoms ( Fig. 1 ). This hypothesis suggests that components of the hypersensitivity immune cascade may play a vital role in FGIDs. A key component of this pathway is the eosinophil, and this review explores a potential role for eosinophils and associated components of this pathway in FGIDs, specifically irritable bowel syndrome (IBS) and functional dyspepsia (FD), which have been principally studied.
Eosinophils and Functional Gastrointestinal Disorders
The gut is an active immune organ that is the first line of defense for many insults, such as allergens in food, drugs, viruses, bacteria, and parasites. The authors surmise that altered immune function, by way of infection or hypersensitivity on a background of genetic predisposition and environment, triggers symptoms ( Fig. 1 ). This hypothesis suggests that components of the hypersensitivity immune cascade may play a vital role in FGIDs. A key component of this pathway is the eosinophil, and this review explores a potential role for eosinophils and associated components of this pathway in FGIDs, specifically irritable bowel syndrome (IBS) and functional dyspepsia (FD), which have been principally studied.
Eosinophils in the Gastrointestinal Tract
Eosinophils are a normal constituent throughout the gastrointestinal tract (GIT), except in the squamous mucosa of the esophagus. These cells have a defined role in physiologic and pathologic function. In the gut mature eosinophils originate from hemopoietic stem cells in the bone marrow; recruitment is regulated by interleukin (IL)–5, IL-3, and granulocyte macrophage colony-stimulating factor (GM-CSF) to induce migration to the gut. Eosinophils are effector cells, which release secretory granules and have an immunoregulatory function by way of cytokines and release and presentation of antigens .
What is the Normal Eosinophil Count in the Gut?
Eosinophils have a characteristic morphology with a bright eosinophilic cytoplasm and a bilobed nucleus. They are easily recognizable in biopsies and therefore counts of eosinophils can be performed on routine hematoxylin and eosin stained sections.
Counting of eosinophils is critical at all sites to discriminate normal from a pathologic state. In the esophagus, eosinophils are evoked in acid reflux and to distinguish this from eosinophilic esophagitis, the cutoff level is conventionally considered to be at least 15 per high power field (HPF), although variable cutoffs have been used . In gastric mucosa, eosinophilia is often seen in Helicobacter pylori infection but typically not in functional dyspepsia . In H pylori –negative adult subjects, the mean gastric eosinophil counts were 10/5HPF in antral and body mucosa . In the duodenum, eosinophilia has been noted in children and adults who have functional dyspepsia, defined as greater than 10/HPF for children and greater than 22/5HPF with or without clusters of eosinophils at the base of glands for adults . Normal duodenal counts were defined by less than 10/HPF in children and 19/5HPF in adults in these studies based on control values . In the cecum, eosinophils are more numerous in the normal state, and up to 40/HPF have been suggested to be normal at this site . Colonic eosinophilia has also been associated with IBS, inflammatory bowel disease (IBD), and food allergy in children, and mean eosinophil numbers per crypt (±standard deviation) have been reported to be 34.8 (±17.1) in IBD, 21.3 (±8.8) in IBS, and 25.4 (±16.7) in food allergy . In this study there was no variation between colonic sites. Geographic variation in colonic eosinophilia in routine biopsies, in which eosinophils in the intercryptal space were counted, has been noted in the United States, where those in southern states had a higher count than the northern states .
Few studies have consistently counted in the same manner in normal or diseased subjects so establishing true normal values is difficult. There are relatively few studies to adequately address the normal counts throughout the gut, particularly the upper gut. Because subtle changes in numbers may be significant , it is important therefore for histopathologists to count eosinophils in the gastrointestinal sites and have data on local normal controls to define abnormality.
Eosinophils, Atopy, Allergy, and Functional Gastrointestinal Disorders
It has been recognized that gastrointestinal symptoms often occur in children who have allergy and atopy , and IBS is significantly more prevalent in adult patients who have allergic diseases, such as asthma and allergic rhinitis . Key cells in the allergy cascade are eosinophils. Small bowel biopsies from patients who have asthma and allergic rhinitis show accumulation of eosinophils, T-cells, mast cells, macrophages, and increased expression of the pro-allergic cytokines IL-4 and IL-5, which are also observed in the inflammatory reaction in the airways .
Allergy to foodstuffs is a common patient perception. In a study from Germany, 32% of patients attending outpatients complained of adverse reactions to food as a cause of their abdominal symptoms. In 14%, the diagnosis of intestinal food allergy was suspected by an elevated total IgE, specific IgE against food antigens, peripheral eosinophilia, responsiveness to cromoglycate, and clinical signs of atopic disease. In only 3% (one tenth of the original cohort), however, was the diagnosis confirmed by endoscopic allergen provocation or elimination diet and re-challenge .
Eosinophils in the lower intestine of patients who have documented food allergy show characteristic features with regard to morphology, distribution, and functional behavior to IgE receptor stimulation. By immunohistochemical analysis of eosinophil peroxidase, there was a highly significant increase of eosinophils throughout the lower GI tract in samples obtained from patients who had food hypersensitivity–related diarrhea compared with control subjects .
Although there is reasonable evidence for a role for food allergy in provoking GI symptoms in eosinophilic gastroenteritides, there is emerging evidence that food allergy may be also important in FGIDs. A recent systematic review of this subject concluded that food allergy may be a factor contributing to the development of some FGIDs and food-specific IgE antibodies can mediate hypersensitivity reactions in patients who have IBS by sensitizing mucosal mast cells .
It has also been shown in clinical trials with exclusion diets that there is the potential to reduce symptoms in a subgroup of patients who have IBS with a clear history of adverse food reactions. These specific foods included wheat, beef, pork, and lamb in a study from the United Kingdom . A second study from Australia of patients who had IBS and FD suggested wheat, crab, and shrimp allergy in IBS, whereas patients who had FD had high antibody titers to soybean and egg . It was concluded that these findings did not contribute to the pathogenesis of FD and IBS; however, the method did not include biopsy of the GI tract to evaluate eosinophils and mast cells.
Eosinophils, Infection, and Functional Gastrointestinal Disorders
Bacterial infections have been implicated as a trigger of FGIDs, particularly in the development of IBS, in which a specific postinfectious type is best recognized . Postinfectious FD also seems probable, because acute onset of FD has been described following Salmonella infection in Spain . Another study noted that 17% of patients who had acute-onset FD describe a preceding infective episode .
The bacterial species responsible for postinfectious IBS include Salmonella , Shigella , and Campylobacter jejuni . These patients have an initial response with an increase in the CD3 and CD8 T lymphocyte population and a striking increase in serotonin containing enterochromaffin cells . Although these changes may decline in most subjects, those who have persistent symptoms may continue to have persistent but subtle cellular changes. Patients who develop postinfectious IBS when assessed up to 3 months following infection still had a 20% increase in enterochromaffin cells, which contain serotonin .
In FD, meta-analyses of trials show a small benefit of eradication of the gastric bacteria H pylori . In dyspepsia in the absence of alarm features and in FD, a policy of test and treat for H pylori is generally recommended; the number needed to successfully treat FD is 15, which suggests that this is not the sole cause of FD and no consistent disturbance of upper GI motility or sensory disturbances have been found to be linked to H pylori infection .
Few studies have addressed the immune pathways in FGIDs. Notably, in eosinophil infiltration can be a major component after bacterial infection. Gastric eosinophil infiltration has been reported to be significantly higher in patients who have serum CagA positivity for H pylori . A study of subjects who have FD showed gastric eosinophilia was predominant only in H pylori infection . In colonic and rectal biopsies of patients who had IBS, eosinophils were not noted to be increased compared with controls, although these were not typed as to whether these were postinfection cases . Although not all patients who have FGIDs have overt atopy, allergy, or history of infection, we have postulated that this type of inflammatory mechanism, and in particular the eosinophil, may provide potential answers to the pathophysiology of certain FGIDs .
Eosinophils in the Duodenum in Functional Dyspepsia and Irritable Bowel Syndrome
It is postulated that the pathophysiology of FD is linked to duodenal dysmotility with distension and sensitivity to luminal contents . Biopsy of the duodenum to ascertain pathology is, however, not routine practice and there are few studies that have focused on duodenal histology as a potential target for investigation. A study of pediatric patients who had dyspepsia with duodenal biopsies found that in 71% had duodenal eosinophilia. Following treatment with H1 and H2 antagonist therapy, on repeat biopsy this was reduced and abdominal pain decreased or disappeared . We have shown that duodenal eosinophilia is associated with FD in adults . In our population-based study of 1000 normal subjects, not actively seeking health care, the prevalence of reflux symptoms, dyspeptic symptoms, and irritable bowel symptoms was 40%, 38%, and 30% respectively . There was a significant duodenal eosinophilia in subjects who had FD compared with controls and it was also noted in subjects who had FD that eosinophils formed clusters at the base of duodenal mucosal crypts . These clusters of eosinophils in the duodenal bulb and second part were present more often in subjects who had FD compared with controls (respectively 51% versus 21%; 62% versus 12.5%). Furthermore, degranulation of eosinophils was seen only in subjects who had FD in a subset analysis (7 of 15 cases, 47% versus none in the 5 controls). In subjects who had IBS from the same cohort, however, duodenal eosinophilia was not observed, suggesting that duodenal eosinophilia is linked to FD but not IBS .
Eosinophil Function in the Gut
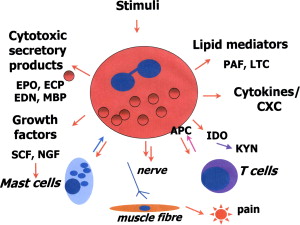
Eosinophils store active immunologic mediators in two intracellular compartments, large granules and small secretory granules. These active mediators are cationic proteins, growth factors, cytokines, chemokines, lipid mediators, and neuromodulators. The large granules contain four primary cationic proteins: eosinophil peroxidase (EPO), major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN); these are cytotoxic secretory products .
The ability of eosinophils to show diversity in disease, for example by differing modes of degranulation , may be key to a role in the FGIDs. In a study of FD, eosinophils accumulated at the base of crypts and degranulation of MBP was shown by immunocytochemistry in those who had dyspepsia, but not in control subjects .
The interaction of eosinophils with other cells in the hypersensitivity cascade may be important in their role in FGIDs. In allergic inflammation, a combination of Th2 cell and cytokines (IL-4, IL-5, and IL13) and mast cell activity seems to be responsible for the initiation of eosinophil activity . This response is sustained because it has been shown that tissue eosinophils can survive for 2 weeks in vitro . IL5 and eotaxin selectively regulate eosinophil trafficking . The Th2 cytokines (tumor necrosis factor, IL4, and IL13) up-regulate eotaxin production . Eotaxin is produced locally and recruits eosinophils to the intestine with accumulation in Peyer patches . Although eotaxin-1 has been noted to play a role in eosinophilic esophagitis in mice, eotaxin-1–deficient mice only developed modestly attenuated disease . Esophageal eotaxin-3 mRNA and protein levels strongly correlated with tissue eosinophilia and mastocytosis in eosinophilic esophagitis in human subjects who have characteristic gene arrays, and a single-nucleotide polymorphism in the human eotaxin-3 gene was associated with disease susceptibility .
This susceptibility to eosinophil accumulation because of a genetic predisposition may provide a link to a genetic susceptibility to FGIDs. Twin studies support a genetic predisposition to FGIDs, although FD has not been adequately evaluated . An association between the CC genotype of GNβ3 and functional dyspepsia has been observed in two independent studies, which may reflect an altered immunophenotype in subjects prone to dyspepsia .
Eosinophils also secrete growth factors, including nerve growth factor (NGF), which maintains sympathetic neurons and promotes mast cell survival and activation. NGF is also produced by mast cells and has a role in resolution of tissue healing. . Other major mediators secreted by eosinophils are the lipid mediators, leukotrienes (LTC) and platelet-activating factors (PAF), chemokines, and cytokines .
Eosinophil Interactions in Gut Mucosa
Eosinophils and T Cells
Eosinophils communicate with T cells in a bidirectional manner and activate T cells by serving as antigen-presenting cells (APCs). Eosinophils can also regulate T-cell polarization through synthesis of indoleamine 2,3-dioxygenase (IDO). IDO catalyzes the oxidative metabolism of l -tryptophan to N-formylkynurenine (KYN), a regulator of Th1/Th2 balance . KYN is subsequently converted to a range of metabolites, including xanthurenic acid and the neurotoxin quinolinic acid. IDO activity is induced in response to interferon-γ and requires heme as a cofactor. Recent studies have suggested that, in vivo, IDO expression by APCs is responsible for inhibiting T-cell proliferation, and may be involved in immune tolerance .
In a study of subjects who had FD, the number of duodenal intraepithelial lymphocytes (IELs) was similar in patients who had FD and healthy controls. A careful analysis of activation markers on IELs showed CD95/Fas and HLA-DR were expressed by a significantly smaller percentage of duodenal CD3+ IELs in H pylori –negative patients who had FD defined according to Rome II criteria . These lower ratios of CD95/Fas and HLA-DR expressing duodenal IELs in dyspeptic patients may reflect the presence of an altered population of primed lymphocytes . Possibly these may be regulated by the increased eosinophils as seen in FD .
Eosinophils and Mast Cells
Mast cells are bone marrow–derived granulocytes, also normally present in the small bowel, which contribute to a variety of immune responses. Mast cells are activated in stress , without allergic degranulation . This finding may provide an all-important link with stress-related symptoms of FGIDs. Mast cells have been studied in FGIDs, particularly IBS, wherein they are increased in the jejunum , ileum , and cecum , and lie in close proximity to enteric nerves in colonic and rectal mucosa . In FD, gastric mast cells may be increased in both H pylori– negative and –positive patients .
Mast cells can be activated through eosinophil-derived MBP by way of a similar pathway to the neuropeptide substance P mast cell activation pathway. MBP and substance P may both directly activate the mast cell Gi3 protein that controls degranulation . Eosinophils express various growth factors, including stem cell factor (SCF), which promote mast cell survival, proliferation, maturation, and mediator release. It is speculated that the production of SCF by eosinophils is stimulated by mast cell chymase ; eosinophil-derived NGF further influences mast cell growth and survival by way of a specific TrkA NGF receptor expressed by mast cells . Mast cells and eosinophils are thus codependent; initially mast cells can induce eosinophils into the mucosa, and these in turn activate mast cells by way of mediators and growth factors, causing proliferation, maturation, and degranulation. Mast cells in the presence of eosinophil-derived growth factors demonstrate resilience to death induced by death receptors .
The mast cell has been principally studied in IBS. The mast cells are not only increased but show greater proximity to nerves, and this has been positively correlated with the severity and frequency of patients’ abdominal pain . Mast cells express receptors for the endocrine and paracrine messengers released from associated nerve endings and also have the ability to produce, store, metabolize, and release relevant molecules that modulate neural responses . Substance P, released from nerve endings, activates mast cells. The mast cells release a mediator, which in turn results in neural activation, promoting intestinal secretion . Nonactivated human mast cells that have not formed synaptic associations with nerve fibers do not respond to neuropeptides, such as substance P .
An interaction between mast cells and the CNS in rats has been demonstrated by pairing an audiovisual cue with an antigen challenge, thereby degranulating mast cell protease II. This interaction occurred predominantly in the intestinal mucosal mast cells . In mast cell hyperplasia and inflammation, afferent vagal nerves penetrate jejunal mucosa and synapse with intestinal mucosal mast cells . If truncal vagotomy is performed, the concentration of mucosal mast cells decreases and, conversely, stimulation of the cervical vagus nerve leads to an increase in mast cell concentration and histamine content of jejunal mucosa . This finding provides important evidence for a CNS link to hypersensitivity ( Fig. 2 ).