Eosinophilic esophagitis in adults is a disease characterized by eosinophilic infiltration of the esophageal mucosa and symptoms of long-standing solid food dysphagia and food impactions. First described in 1978, this syndrome is being recognized increasingly in the developed world, with multiple case series reported from the United States, Europe, and Australia during the past decade. Diagnosis requires the presence of greater than or equal to 15 eosinophils/high-power field on esophageal biopsies. Successful treatment in adults has been reported with the use of systemic and topical swallowed steroids. Endoscopic treatment has been associated with increased an risk for tears and perforations.
Eosinophilic esophagitis (EE) is a disease characterized by eosinophilic infiltration of the esophageal mucosa, and is associated with a clinical syndrome of dysphagia and food impaction (FI) in adults. First described in 1978 , this syndrome is being recognized increasingly in the developed world, with multiple case series reported from the United States, Europe, and Australia during the past decade . It is unclear if the increasing recognition of the disease is due to a truly increasing incidence or is a reflection of increased recognition by pathologists and clinicians. Endoscopic features suggestive of EE have also been described, including the presence of rings, longitudinal furrows, and mucosal fragility, although a proportion of patients may have normal-appearing mucosa. Recently, the specificity of these endoscopic findings has been questioned . Diagnosis is established by the presence of eosinophilic infiltration of the esophageal mucosa; different thresholds have been used by various investigators, ranging from more than 15 eosinophils/high-power field (HPF) to more than 24 eosinophils/HPF. A recent consensus statement proposed the use of more than 15 eosinophils/HPF as the diagnostic criteria for EE in the proper clinical context . The cause of EE remains unclear, with allergic (allergies to food or aeroallergens) and immunologic mechanisms being proposed . Successful treatment in adults has been reported with systemic and swallowed topical steroid preparations (in case series and randomized controlled trials) and with oral leukotriene inhibitors in small case series . Endoscopic dilation alone, and in combination with medications, has also been reported as a treatment modality in patients who have EE . The natural history and clinical course of EE in adults is thought to be characterized by recurrent symptoms but remains poorly defined.
Epidemiology
Data on the epidemiology of EE in adults remain scarce, particularly in the United States. Straumann and Simon reported the increasing prevalence of EE diagnosed in adults in Olten County, Switzerland. Olten County is a defined geographic area with a stable population of 100,000, with a single gastroenterologist and a single pathologist providing care. The investigators provided estimates from 1989 to 2004. They reported an average annual incidence of 1.48 cases/100,000 population (range, 0–6) with a marked increase in cases during the past few years of this time period. The prevalence of EE progressively increased over this time period, to 23 cases/100,000 population. The investigators considered this figure to be an underestimate, given that it represented only severely symptomatic patients.
The first truly population-based estimate of esophageal eosinophilia was provided from northern Sweden , where 1000 randomly selected subjects from two counties who had completed an abdominal symptom questionnaire underwent endoscopy with biopsies (taken 2 cm above, and at, the gastroesophageal junction). Biopsies were taken by three trained gastroenterologists and were interpreted by two gastrointestinal pathologists. The investigators classified patients into those with definite EE (≥20 eosinophils/HPF), probable EE (15–19 eosinophils/HPF), and possible EE (5–14 eosinophils/HPF). They found that 4 patients had definite EE, 7 had probable EE, and 25 had possible EE. In addition, they reported that 48 patients in all had eosinophils present in their esophageal biopsies; hence, the prevalence of EE by the current consensus definition of EE (>15 eosinophils/HPF) was 1.1%. Of the 4 patients who had definite EE in this study, 3 had symptoms of gastroesophageal reflux disease (GERD) and 1 was completely asymptomatic. Given the cross-sectional nature of this study, estimates of the incidence of EE could not be assessed.
Secular trends in the epidemiology of EE were assessed in Olmsted County, Minnesota, during the past 3 decades (1976–2006) in a retrospective study. All cases of EE diagnosed between 1976 and 2006 were identified using the resources of the Rochester Epidemiology Project. All esophageal biopsies with any evidence of eosinophilic infiltration were reviewed by a single pathologist. Patients presenting with unexplained FI needing endoscopic therapy were included as probable surrogates of EE, to maximize case identification. The clinical course of all patients was also defined using medical records and, prospectively, by using a telephone questionnaire. A total of 3456 patient charts were reviewed; 82 patients who had EE and 80 patients who had idiopathic FI were identified. The incidence of EE increased significantly during the past 3 decades (from 0.86 cases [95% CI, 0.15, 1.56]/100,000 population/year from 1976 to 1985, to 8.78 cases [95% CI, 7.19, 10.37]/100,000 population/year from 1996 to 2006). The prevalence of EE was 104.7 cases (87.5, 122.0)/100,000 population as of 1/1/2007 in Olmsted County. In this study, the prevalence and incidence of EE appear to be higher than previously reported. The incidence of EE has increased significantly during the past 3 decades, perhaps indicating the presence of an as-yet-unidentified cause of EE in the environment.
Estimates of the prevalence and incidence of EE in pediatric patients have been reported by investigators from Hamilton County, Ohio. The incidence ranged from 0.9 cases/10,000 population in 2000 to 1.28 cases/10,000 in 2003, with the prevalence in 2003 estimated to be 4.29 cases/10,000 population; these estimates were higher than estimates of Crohn’s disease in the pediatric population . Lower estimates of prevalence were reported in a pediatric population from western Australia by Cherian and colleagues (0.89 cases/10,000). A common feature among all case series reported in the literature is the male preponderance of cases and the diagnosis of the disease in younger patients. Other factors influencing EE, such as racial predilection or socioeconomic status, have yet to be evaluated.
Natural History
The natural history of EE in adults was perhaps best described by Straumann and colleagues , who followed 30 adults who had EE (>24 eosinophils/HPF) with periodic dilations alone during a mean of 7.2 years. They reported that quality of life was affected in a severe manner in only 1 patient and in a minor manner in 15. Nutritional status was not compromised in any patient. Of the 30 patients, only 11 were treated with endoscopic dilation; of these, 4 had repeated dilations and 7 required only a single dilation. The remaining 19 patients were monitored only. Patients who had peripheral blood eosinophilia and severe endoscopic abnormalities were more likely to relapse than those who did not. Although fibrosis of the lamina propria was noted in later biopsies, no progression to deeper layers or to other parts of the gastrointestinal tract was observed. No patient developed esophageal carcinoma or hypereosinophilic syndrome. Hence, EE does not appear to influence life expectancy. In another study, reported from the Mayo Clinic in abstract form , follow-up of 10 patients who had EE 3 years after diagnosis using a mailed questionnaire revealed recurrent solid food dysphagia occurring at least once a week in 60% of patients. All had received retreatment with inhaled steroids, and 70% had received endoscopic dilation despite medical treatment. The clinical course of patients who had EE in Olmsted County was characterized by recurrent symptoms in 40% of patients responding to medical or endoscopic treatment, with EE also appearing to be a recurrent relapsing disease in a substantial proportion of patients.
Pathogenesis
Debate continues on the etiopathogenesis of EE, with allergic and immunologic mechanisms being proposed and investigated , based on the substantial proportion of adult patients who have associated food, inhalant, and seasonal allergies (40%–60%) (although this appears to be somewhat lower than in children, where up to 80% have been reported to have an allergic diathesis ). Specific IgE testing, skin prick testing, and atopy patch testing have been reported to be positive in variable (32%–80%) proportions of adults and children with EE . On the basis of allergy testing results, it is thought that EE may be a result of IgE-mediated and non–IgE-mediated reactions . In addition, the relationship between EE and GERD remains to be defined , given the presence of esophageal eosinophilia in patients who have GERD (albeit to a lesser degree).
Two possible mechanisms of antigen sensitization have been proposed in EE. One is an initial esophageal sensitization to a food antigen, resulting in EE when esophageal re-exposure to the same antigen results; an alternative hypothesis is that initial bronchial sensitization followed by esophageal re-exposure leads to EE. This latter mechanism was supported by experiments conducted by Mishra and colleagues , when they exposed mice to Aspergillus fumigatus intranasally and intragastrically. Only mice exposed to intragastric A fumigatus developed esophageal eosinophilia. A bronchial esophageal connection was also supported by experiments in which airway delivery of interleukin (IL)-13 promoted the development of EE . EE appears to be associated with a Th2-type immune response; increased levels of eosinophil-active Th2 cytokines (such as IL-4, IL-5, and IL-13) and mast cells have been described in the esophagus in patients who have EE. Experimental models of EE can be induced in mice by means of allergen exposure, especially in the respiratory tract after mucosal or epicutaneous sensitization, and by means of overexpression of Th2 cytokines (IL-5 and IL-13) .
The esophagus is normally devoid of eosinophils. In patients who have EE, recruitment of eosinophils to the esophagus is thought to be mediated by IL-5 and eotaxin. IL-5 is a cytokine produced by T-helper (type 2) lymphocytes, which can prime eosinophils to react to chemoattractants such as eotaxin (an eosinophil chemotactic factor, also known to be overexpressed in patients who have EE Ref. ) ( Fig. 1 ). IL-13 has also been shown to be crucial in the development of EE. In sensitized individuals, allergen exposure can lead to IgE-mediated mast cell degranulation, which leads to the production of chemokines, histamine, and eosinophilic chemoattractants. These factors then induce eosinophil migration and degranulation, releasing products such as major basic protein (MBP), eosinophil cationic protein, and eosinophil-derived neurotoxin. These products can cause tissue damage, edema, and chronic inflammation, which, if prolonged, can lead to fibrosis . In addition, these cationic proteins released by eosinophils set up a positive feedback loop by causing further mast cell degranulation, which leads to greater eosinophil recruitment to the esophagus and further release of eosinophil granule products . The effect of eosinophil products such as MBP on smooth muscle has also been described, with muscarinic M2 receptors mediating smooth muscle contraction. This finding may explain the ability of eosinophilic infiltration of the esophagus to lead to dysphagia and FI, analogous to bronchoconstriction in asthma .
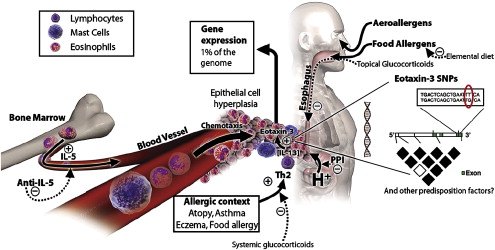
Familial clustering of EE has been reported by some investigators . Whether this clustering reflects a true genetic predilection or a common environmental exposure remains unclear. Support for a genetic basis of EE comes from a study that found that the gene encoding for eotaxin 3 was the most highly induced gene in EE patients when compared with healthy controls . Seasonal variations in the diagnosis and severity of symptoms of EE have been reported in adults and children , rendering support to the hypothesis that environmental allergens play a role in the pathogenesis of EE.
Pathogenesis
Debate continues on the etiopathogenesis of EE, with allergic and immunologic mechanisms being proposed and investigated , based on the substantial proportion of adult patients who have associated food, inhalant, and seasonal allergies (40%–60%) (although this appears to be somewhat lower than in children, where up to 80% have been reported to have an allergic diathesis ). Specific IgE testing, skin prick testing, and atopy patch testing have been reported to be positive in variable (32%–80%) proportions of adults and children with EE . On the basis of allergy testing results, it is thought that EE may be a result of IgE-mediated and non–IgE-mediated reactions . In addition, the relationship between EE and GERD remains to be defined , given the presence of esophageal eosinophilia in patients who have GERD (albeit to a lesser degree).
Two possible mechanisms of antigen sensitization have been proposed in EE. One is an initial esophageal sensitization to a food antigen, resulting in EE when esophageal re-exposure to the same antigen results; an alternative hypothesis is that initial bronchial sensitization followed by esophageal re-exposure leads to EE. This latter mechanism was supported by experiments conducted by Mishra and colleagues , when they exposed mice to Aspergillus fumigatus intranasally and intragastrically. Only mice exposed to intragastric A fumigatus developed esophageal eosinophilia. A bronchial esophageal connection was also supported by experiments in which airway delivery of interleukin (IL)-13 promoted the development of EE . EE appears to be associated with a Th2-type immune response; increased levels of eosinophil-active Th2 cytokines (such as IL-4, IL-5, and IL-13) and mast cells have been described in the esophagus in patients who have EE. Experimental models of EE can be induced in mice by means of allergen exposure, especially in the respiratory tract after mucosal or epicutaneous sensitization, and by means of overexpression of Th2 cytokines (IL-5 and IL-13) .
The esophagus is normally devoid of eosinophils. In patients who have EE, recruitment of eosinophils to the esophagus is thought to be mediated by IL-5 and eotaxin. IL-5 is a cytokine produced by T-helper (type 2) lymphocytes, which can prime eosinophils to react to chemoattractants such as eotaxin (an eosinophil chemotactic factor, also known to be overexpressed in patients who have EE Ref. ) ( Fig. 1 ). IL-13 has also been shown to be crucial in the development of EE. In sensitized individuals, allergen exposure can lead to IgE-mediated mast cell degranulation, which leads to the production of chemokines, histamine, and eosinophilic chemoattractants. These factors then induce eosinophil migration and degranulation, releasing products such as major basic protein (MBP), eosinophil cationic protein, and eosinophil-derived neurotoxin. These products can cause tissue damage, edema, and chronic inflammation, which, if prolonged, can lead to fibrosis . In addition, these cationic proteins released by eosinophils set up a positive feedback loop by causing further mast cell degranulation, which leads to greater eosinophil recruitment to the esophagus and further release of eosinophil granule products . The effect of eosinophil products such as MBP on smooth muscle has also been described, with muscarinic M2 receptors mediating smooth muscle contraction. This finding may explain the ability of eosinophilic infiltration of the esophagus to lead to dysphagia and FI, analogous to bronchoconstriction in asthma .
Familial clustering of EE has been reported by some investigators . Whether this clustering reflects a true genetic predilection or a common environmental exposure remains unclear. Support for a genetic basis of EE comes from a study that found that the gene encoding for eotaxin 3 was the most highly induced gene in EE patients when compared with healthy controls . Seasonal variations in the diagnosis and severity of symptoms of EE have been reported in adults and children , rendering support to the hypothesis that environmental allergens play a role in the pathogenesis of EE.
Clinical Manifestations
Patients who have EE tend be young men, with a male gender preponderance (a male/female ratio of 3:1 was reported in one meta-analysis Ref. ). Most patients are diagnosed in the third or fourth decade of life. The most common presenting symptom in adults is dysphagia to solids (60%–90%). FI is also a common presenting manifestation, with 50% to 60% of patients reporting episodes. Two prospective studies have reported that 50% to 55% of patients presenting to the emergency room with FI have EE, based on esophageal biopsies . In addition, symptoms of GERD (heartburn and acid regurgitation) are also common in adults who have EE, from 24% in a meta-analysis to 50% in a prospective study of patients who had dysphagia . Some patients who have EE may also present with acid reflux symptoms that do not respond to medical treatment with proton pump inhibitors. Despite the presence of acid reflux symptoms, most patients who have EE (82%) have normal or negative ambulatory pH studies . Chest pain, abdominal pain, diarrhea, and weight loss are some of the other symptoms reported by patients diagnosed with EE. This pattern of presenting symptoms in adults differs from that of children, who present with more nonspecific symptoms such as poor feeding, vomiting, regurgitation, and failure to thrive. Older children and adolescents present with symptoms similar to those of adults. Symptoms in adults are compared with those in children in Box 1 .
Adults
Dysphagia
Food impaction
Heartburn/acid reflux not responsive to medical treatment
Chest pain
Children
Feeding aversion
Vomiting, regurgitation
Failure to thrive
GERD symptoms not responsive to medical treatment
Abdominal pain
Chest pain
Dysphagia, food impaction
Ten percent to fifty percent of studies in adults have reported varying degrees of peripheral eosinophilia . The degree of eosinophilia reported has been modest and is complicated by studies using different thresholds to define eosinophilia. A recent meta-analysis reported that 30% of patients (82/266 patients from 19 studies) had peripheral eosinophilia .
Diagnosis
Endoscopic Manifestations
Several typical endoscopic manifestations of EE have been described in case series of EE, including esophageal rings, which lead to “trachealization” or “felinization” of the esophageal mucosa ( Fig. 2 A); raised white specks, which may represent eosinophilic microabscesses ( Fig. 2 B); longitudinal furrows ( Fig. 2 C); whitish exudates; and “crepe paper” mucosa, which refers to a friable mucosa that tears by the mere passage of the endoscope. Strictures can be located in the proximal, middle, or lower third of the esophagus. Case series also describe a substantial proportion of patients who have EE as having normal esophageal mucosa on endoscopy (10%–33%) . A prospective study on outpatients presenting with dysphagia found that 10% of patients who had a “normal endoscopy” had evidence of EE on midesophageal biopsies, supporting the argument for taking esophageal biopsies in all patients who have dysphagia and a “normal” endoscopic examination . The same study also found that the typical endoscopic manifestations of EE have a poor predictive value for a diagnosis of EE, with only 38% of patients who had typical endoscopic features suggestive of EE meeting the histologic criteria for EE on midesophageal biopsies. However, the presence of more than one endoscopic feature of EE increased the likelihood of EE on esophageal biopsies in the same study .
Esophageal Biopsies
Site
The recent consensus statement on the diagnosis and management of EE states that “multiple biopsy specimens should be obtained from different esophageal locations along the length of the esophagus” to confirm a diagnosis of EE in all patients who have suspected EE, “regardless of the gross appearance of the esophageal mucosa” . Multiple prior studies have based their diagnosis of EE on biopsies obtained from either the proximal or the distal esophagus. These studies have demonstrated a marked variability in the degree of eosinophilia in biopsies taken from different levels of the esophagus, with levels from the distal esophagus being numerically greater than those from the proximal esophagus . Gonsalves and colleagues performed a subgroup analysis in 20 out of 61 patients who had proximal and distal biopsies available; they found that 4 patients (20%) met the histologic criteria for EE only on distal biopsies. In contrast, in a large pediatric study, proximal esophageal biopsies demonstrated 100% sensitivity in making a diagnosis of EE, when compared with distal esophageal biopsies.
Number
In an elegant study, investigators modeled the sensitivity of diagnosis by varying the number of biopsies , with a diagnostic threshold of 15 eosinophils/HPF. The sensitivity of one biopsy specimen was 55% and increased to 100% with five biopsy specimens ( Fig. 3 ), which led the consensus statement on the diagnosis of EE to recommend that “multiple” biopsy specimens be taken. At the authors’ institution, they recommend that at least four biopsies be taken from the esophagus at different levels, to maximize sensitivity. Despite multiple biopsies, false-negative results may occur because of patchy distribution of the eosinophilic infiltrate, other medications the patient may be taking (such as systemic or inhaled steroids and leukotriene inhibitors), or involvement of deeper esophageal layers (muscularis propria) by the eosinophilic inflammation .









