This article provides an overview of the evaluation and treatment of female factor infertility. It reviews the physiology of female reproduction and subsequently discusses pertinent findings in the evaluation and treatment of female infertility that are relevant to a urologist treating the male partner. Finally, it provides an overview of current treatment modalities.
Infertility is a disease of couples, and it affects 2.1 million married couples, many of whom seek fertility assistance . Once thought to be a disease of women, it is clear that the cause of infertility in couples seeking advanced reproductive technology can be found in the male partner (18.5%), female partner (50%), both partners (18.4%), or neither partner (unexplained infertility, 12%). In 2006, 8% of married childless couples sought infertility services, and 15% of all women report having used some infertility service in the past. It behooves urologists who care for infertile men to be acquainted with female fertility. This article provides an overview of the causes for and treatment of female factor infertility.
Female reproductive physiology
In contrast to spermatogenesis, oogenesis begins in utero. By the fifth month of gestation, mitosis is complete and the peak number of oocytes is achieved. Barring any causes of ovarian failure, a pubertal girl has approximately 400,000 oocytes resting in primordial follicles containing a single layer of granulosa cells. During embryologic oogenesis, oocyte development arrests in the diplotene phase of the first meiotic division and does not resume meiosis until after ovulation. The process of ovulation requires that the follicle develop from a primordial follicle to a tertiary follicle over approximately three menstrual cycles. Only the final phase of folliculogenesis is hormone dependent. The process continues throughout postpubertal life even in the presence of cycle disruptors such as oral contraception or pregnancy. Follicles that reach the hormone-dependent stage at a time asynchronous with the menstrual cycle become atretic. Subsequently, only 300 to 400 follicles ovulate in a woman’s lifetime.
The first day of the menstrual cycle is marked by the first day of bleeding. At this point, ovarian and pituitary hormone productions are at their nadir, and multiple morphologically identical antral follicles are present. Under the control of gonadotropin-releasing hormone from the hypothalamus, the anterior pituitary begins to release follicle-stimulating hormone (FSH) to begin hormone-dependent follicular growth. Cumulus expansion of the granulosa cells occurs as the oocytes compete to be the dominant follicle. Although selection of the dominant follicle is poorly understood, it is clear that the intrafollicular environment plays a significant role. The follicular fluid of the dominant follicle has significantly higher levels of estrogen than its competing cohort. There is also a greater concentration of luteinizing hormone (LH) receptor on the theca cells and FSH receptor on the granulosa cells. Several authors have theorized that the ability to recruit steroid receptors and produce estrogen is the major determinant of dominance . Human animal data suggest that insulin-like growth factor (IGF) plays a major role in dominance selection; the Igf null mouse does not undergo hormone-dependent folliculogenesis . In the bovine model, the dominant follicle has a low concentration of IGF binding proteins and an elevated concentration of pregnancy-associated plasma protein-A and insulin-like growth factor binding protein (IGFBP) proteolytic enzyme . A significant association between intrafollicular pregnancy-associated plasma protein-A and estradiol levels suggests that pregnancy-associated plasma protein-A plays a role in dominance selection in humans . IGF is synergistic with FSH to augment estradiol production . Data suggest that increase in intrafollicular-free IGF concentration is associated with dominance.
Clearly, the induction of estradiol is a key component of the follicular cycle. Initially, this estrogen has a negative feedback on the hypothalamus and pituitary. After follicular dominance is determined, however, estradiol exerts positive feedback that leads to the surge of LH. Again, the mechanisms involved in this surge are unclear. In addition to inducing ovulation, LH is required for the resumption of meiosis . At the time of ovulation, the oocyte completes meiosis I and arrests in the metaphase of meiosis II. Meiosis is completed at the time of fertilization. The mechanisms involved in meiosis resumption are under intense study by researchers interested in in vitro maturation of follicles for preservation of fertility.
After ovulation, the ruptured follicle forms a corpus luteum and produces progesterone to support the endometrium for implantation. Implantation typically occurs 5 to 7 days after ovulation. The early trophoblast cells produce human chorionic gonadotropin to maintain the corpus luteum. In the absence of human chorionic gonadotropin, the corpus luteum involutes and the resultant decrease in estrogen and progesterone results in menses. Corpus luteal hormone production is critical to maintaining the pregnancy during the first 8 weeks of gestation; loss of corpus luteal progesterone production results in abortion.
Evaluation of the female partner
History
A focused history can evaluate for risk factors of infertility and guide further diagnostic modalities and treatment. Risk factors include advancing age, a history of pelvic infections (including salpingitis and appendicitis), severe pelvic pain or endometriosis, and irregular menstrual cycles. One also must ascertain the frequency of intercourse and the use of vaginal lubricants that may be sperm toxic. Of these risk factors, age is the strongest predictor of pregnancy either with or without advanced reproductive techniques. Fecundability begins to decline at age 30, sharply declines after age 35, and is less than 5% after age 40 ( Fig. 1 ). Patients of advancing age are often counseled to begin with more aggressive treatment.
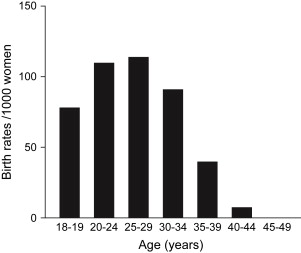
Evaluation of the female partner
History
A focused history can evaluate for risk factors of infertility and guide further diagnostic modalities and treatment. Risk factors include advancing age, a history of pelvic infections (including salpingitis and appendicitis), severe pelvic pain or endometriosis, and irregular menstrual cycles. One also must ascertain the frequency of intercourse and the use of vaginal lubricants that may be sperm toxic. Of these risk factors, age is the strongest predictor of pregnancy either with or without advanced reproductive techniques. Fecundability begins to decline at age 30, sharply declines after age 35, and is less than 5% after age 40 ( Fig. 1 ). Patients of advancing age are often counseled to begin with more aggressive treatment.
Menstrual history
Characterization of a woman’s menstrual regularity may provide insight into the normalcy of ovulation. Normal menstrual cycles range from 24 to 25 days, and menses occurs for 3 to 7 days. In a woman younger than age 35, a history of regular menstrual cycles is highly correlated with the presence of ovulation. This association is strengthened when menses are accompanied by monthly moliminal symptoms, including breast tenderness, bloating, or mood changes. A long cycle is often associated with anovulation. In contrast, a short cycle may be associated with ovulation, with an inadequate follicular phase leading to poor endometrial development or luteal phase deficiency.
Assessment of ovulation
Several tools are available for the assessment of ovulation, including basal body temperature charts ( Fig. 2 ), assessment of mid-luteal progesterone, endometrial biopsy, and urinary LH prediction kits. Each of these tests, with the exception of urinary LH, can only retrospectively inform the user of ovulation. Basal body temperature is effective because progesterone production from the corpus luteum raises core body temperature by approximately 0.6°F, which provides a “biphasic” pattern of temperature. Temperature should be taken every morning, however, which reminds the woman of infertility before starting her day. This reminder is cumbersome and may be emotionally taxing.
Measurement of mid-luteal progesterone is another retrospective method of determining ovulation. Clinicians often use this value to determine the adequacy of the luteal phase; however, data do not support a specific value that correlates with a luteal phase defect. Endometrial biopsy on cycle has long been considered the “gold standard” determinant of ovulation. After ovulation, the endometrium undergoes classic morphologic changes that correlate with the day of the menstrual cycle. It has been suggested the endometrial dysynchrony of two or more days, as determined by endometrial biopsy, is associated with luteal phase defect . Significant inter- and intraobserver variability is present in the evaluation of endometrial biopsies, however . The National Institutes of Health/National Institute of Child Health and Human Development–funded National Cooperative Reproductive Medicine Network recently completed a study on the use of endometrial biopsy. This multicentered trial concluded that the endometrial biopsy cannot discriminate between fertile and infertile women and should not be a routine part of the infertility evaluation .
Urinary LH testing uses an enzyme-linked immunoassay against the beta subunit of LH, which rises abruptly for approximately 18 hours before it peaks. Ovulation typically occurs approximately 36 hours after the onset of the surge. Because the hormone needs to be conjugated before it is excreted, urinary LH predicts ovulation approximately 24 hours in advance, which provides a prospective assay of ovulation that also can be used to time intercourse.
Ovarian reserve testing
Research has established that age is the best predictor of fertility potential. Ovarian reserve testing is a measure of “ovarian aging.” Although studies have used these tests to predict the time to menopause, most studies have had in vitro fertilization (IVF) pregnancy as the outcome measure. Normative measures of ovarian reserve in a noninfertility population have not been established. Tests of ovarian reserve include the measurement of basal hormone levels, dynamic ovarian testing, and sonographic assessment of the ovaries.
Basal hormones that have been measured to estimate ovarian reserve include FSH, estradiol, inhibin, and anti-müllerian hormone. FSH is inversely proportional to the production of the follicular hormones estradiol and inhibin. It follows that as the cohort of developing follicles is reduced, the basal levels of FSH increase. Over the past 20 years, many studies have evaluated the predictive value of FSH, and the results of these studies are highly conflicting. Although most of these studies agree that the FSH level indicates the number of oocytes retrieved from an IVF cycle, several large studies have failed to demonstrate the test’s ability to predict pregnancy, especially in a young patient population. Estradiol traditionally has been measured in conjunction with FSH to ensure that the FSH was drawn during the cycle nadir. Several studies have demonstrated that elevated estradiol level on day three may be independently predictive of poor stimulation, however . Because neither of these hormones is highly predictive of pregnancy, investigators have tested the predictive value of additional hormones. Inhibin B, a hormone made by the granulosa cells, regulates FSH production by negative feedback. Studies suggest that inhibin B levels decrease earlier than changes in estradiol. Although results are conflicting, this hormone may be an earlier predictor of IVF response .
Dynamic tests of ovarian reserve involve challenging the ovaries with a fertility medication, such as clomiphene citrate or gonadotropin . Although predictive of response to IVF, these tests have not been demonstrated to be superior to basal hormone levels in predicting pregnancy. Likewise, ultrasound ovarian assessments, including ovarian volume and antral follicle counts, may predict response to medications. It is important to re-emphasize that although each test of ovarian reserve may help to counsel patients regarding their fertility potential, age remains the strongest predictor of pregnancy.
Pelvic infections and anatomic abnormalities
Pelvic infections, especially gonorrhea and chlamydia, may irreversibly damage the fallopian tubes. These infections are endemic in the United States and have an annual incidence of approximately 1 million new reported cases. Most of the cases are diagnosed in women between the ages of 15 and 24, with an annual incidence of approximately 1 in 35 women . Unfortunately, because chlamydia is asymptomatic in 75% of cases, a large amount of chlamydial infections are not diagnosed. Up to 20% of women with undiagnosed chlamydia develop infertility. Although gonorrhea and chlamydia are the most common infectious causes of tubal damage, any significant pelvic inflammatory condition can result in adhesion formation that affects tubal integrity.
Severe dysmenorrhea may be associated with uterine anomaly, such as subserosal fibroids or outflow tract obstruction. It also may be associated with endometriosis or pelvic adhesions. This finding also leads to specific diagnostic evaluation.
Anatomic assessment
Assessment of the structural integrity of the reproductive tract is an essential component of the fertility evaluation. It can consist of radiologic imaging or surgical evaluation. The most common test for evaluating the uterine cavity and checking tubal patency is the hysterosalpingogram, which is performed by injecting radiopaque dye into the uterus and tubes under fluoroscopic visualization. Uterine abnormalities are outlined by the dye, and tubal obstruction is noted by the absence of free-spill into the peritoneal cavity. In addition to the diagnostic value of the hysterosalpingogram, the test may be therapeutic .
Intrauterine abnormalities also can be identified with saline-infusion sonography ( Fig. 3 ). Because the uterus is a potential space, traditional ultrasonography is not sensitive enough to determine if a lesion is intracavitary. Saline-infusion sonography is performed by injecting saline into the uterus to provide a sonographic window within the endometrial cavity. The sensitivity and specificity of saline-infusion sonography have both been estimated to be 100% when surgery was used as a gold standard . The advent of three-dimensional ultrasonography has improved the diagnostic capabilities of ultrasonography, and several publications have reviewed its technical aspects . Briefly, the operator selects a region of interest, and specialized probes scan through the region in multiple planes. Computer reconstruction assembles the images to create a structure that can be manipulated and viewed in multiple planes. This technique is highly accurate for diagnosing uterine anomalies and intrauterine pathologic conditions, such as septum and synechiae .
MRI is an excellent modality for viewing soft tissues, thereby surpassing the diagnostic ability of CT for imaging uterine abnormalities. It has been reported to have 100% specificity and 80% to 100% sensitivity for evaluating pelvic anomalies . Because it images in multiple planes, MRI is an excellent preoperative assessment before reproductive gynecologic surgeries such as myomectomy and metroplasty.
Clearly, endoscopy can be used to visualize pelvic anatomy. Although radiologic imaging provides information about the pelvic structures, it provides little information about peritubal adhesions, pelvic infection, or endometriosis. The decision to perform laparoscopy as an initial diagnostic modality is based on the clinician’s suspicion of pathologic condition. For example, in a patient with a history of cyclic pelvic pain that suggests endometriosis, laparoscopy may be the best initial evaluation.
Anatomic cervical abnormalities may result in abnormal cervical mucus production. Cervical mucus protects the sperm from the acidic milieu of the vagina and is critical for introducing sperm into the upper genital tract. Patients with extensive cervical surgery have lower fecundity. Some clinicians evaluate preovulatory cervical mucus with a postcoital test. Cervical mucus is evaluated for elasticity, ferning, and the presence of adequate numbers of motile sperm. The postcoital test has not been associated with improving pregnancy outcome, however.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





