Chapter 31 Extraintestinal Endosonography (including Celiac Block)
Lung Cancer
Lung cancer is the leading cause of cancer death in the United States in men and women and has an overall 5-year survival rate of 15%.1,2 Treatment decisions are based on the location and extent of the tumor. The presence of extrapulmonary metastasis is crucial because patients without mediastinal involvement are potential candidates for resection. The distinction between non–small cell lung cancer (NSCLC), which accounts for 80% of tumors, and small cell lung cancer (SCLC), which accounts for 20% of tumors, is important because of the more aggressive nature of SCLC. SCLC is usually classified as limited or extensive disease, although the criteria for these two categories remain controversial.3–5 Although the TNM (primary tumor, regional nodes, metastases) staging system traditionally has not been used in staging SCLC, it is expected to be included in the forthcoming seventh edition of the TNM classification of malignant tumors.6–9 Metastatic disease is detected in 80% of SCLC cases at the time of diagnosis and tends to spread quickly so that surgery is considered less often in SCLC compared with NSCLC. Although highly responsive to radiotherapy and chemotherapy, SCLC usually recurs within 2 years.
In comparison, half of NSCLC cases are localized or locally advanced and can be treated by surgery, the cornerstone of therapy for NSCLC, or with adjuvant therapy with or without resection.10–12 NSCLC, which includes adenocarcinoma, squamous cell cancer, and large cell cancer, continues to be staged using the 2002 International Staging System, which is unchanged from the 1997 revision (Box 31.1).12–14 This section focuses on EUS applications in the diagnosis and staging of NSCLC, although much of what is covered can be applied to SCLC.
Box 31.1
International Staging System for Lung Cancer, 1997 Revision
Primary Tumor (T)
Nodal Involvement (N)
Data from Greene FL, Page DL, Fleming ID, et al, editors: AJCC (American Joint Committee on Cancer) cancer staging manual, ed 6, New York, 2002, Springer-Verlag, pp 167–174; and Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111:1710–1717, 1997.
Staging and Staging Modalities
Mediastinal lymph node metastases are present in nearly half of all patients with NSCLC. Accurate staging of NSCLC is crucial in determining treatment options because the detection of mediastinal lymph node metastasis preoperatively has therapeutic implications. In the absence of distant metastasis, the documentation of mediastinal metastasis is probably the most common deterrent to cure.15–26 The TNM staging system used for lung cancer (see Box 31.1) designates ipsilateral peribronchial, intrapulmonary, or ipsilateral hilar lymph nodes as N1 disease and ipsilateral mediastinal and subcarinal lymph node involvement as N2 disease. Although N2 disease is potentially resectable, most patients with N2 disease receive multimodality treatment. Contralateral lymph node involvement of mediastinal or hilar nodes or either ipsilateral or contralateral scalene or supraclavicular lymph nodes is designated N3 disease, which precludes resection (Table 31.1 and Fig. 31.1, and see Box 31.1).12–14,26,27
Table 31.1 Lymph Node Map Definitions
| Nodal Station | Anatomic Landmarks |
|---|---|
| N2 NODES: ALL N2 NODES LIE WITHIN THE MEDIASTINAL PLEURAL ENVELOPE | |
| 1. Highest mediastinal nodes | Nodes lying above horizontal line at the upper rim of the brachiocephalic (left innominate) vein where it ascends to the left, crossing in front of the trachea at its midline |
| 2. Upper paratracheal nodes | Nodes lying above horizontal line drawn tangential to the upper margin of the aortic arch and below the inferior boundary of the No. 1 nodes |
| 3. Prevascular and retrotracheal nodes | Prevascular and retrotracheal nodes may be designated 3A and 3B; midline nodes are considered to be ipsilateral |
| 4. Lower paratracheal nodes | Lower paratracheal nodes on the right lie to the right of the midline of the trachea between horizontal line drawn tangential to the upper margin of the aortic arch and line extending across the right main bronchus at the upper margin of the upper lobe bronchus and contained within the mediastinal pleural envelope; lower paratracheal nodes on the left lie to the left of the midline of the trachea between horizontal line drawn tangential to the upper margin of the aortic arch and line extending across the left main bronchus at the level of the upper margin of the left upper lobe bronchus, medial to the ligamentum arteriosum and contained within the mediastinal pleural envelope. |
| Researchers may wish to designate the lower paratracheal nodes as No. 4s (superior) and No. 4i (inferior) subsets for study purposes; No. 4s nodes may be defined by a horizontal line extending across the trachea and drawn tangential to the cephalic border of the azygos vein; No. 4i nodes may be defined by the lower boundary of No. 4s and the lower boundary of No. 4 as described previously | |
| 5. Subaortic (aortopulmonary window) | Subaortic nodes are lateral to the ligamentum arteriosum or the aorta or left pulmonary artery and proximal to the first branch of the left pulmonary artery and lie within the mediastinal pleural envelope |
| 6. Paraaortic nodes (ascending aorta or phrenic) | Nodes lying anterior and lateral to ascending aorta and the aortic arch or the innominate artery, beneath line tangential to the upper margin of the aortic arch |
| 7. Subcarinal nodes | Nodes lying caudal to the carina of the trachea but not associated with the lower lobe bronchi or arteries within the lung |
| 8. Paraesophageal nodes (below carina) | Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes |
| 9. Pulmonary ligament nodes | Nodes lying within the pulmonary ligament, including nodes in the posterior wall and lower part of the inferior pulmonary vein |
| N1 NODES: ALL N1 NODES LIE DISTAL TO THE MEDIASTINAL PLEURAL REFLECTION AND WITHIN THE VISCERAL PLEURA | |
| 10. Hilar nodes | Proximal lobar nodes, distal to the mediastinal pleural reflection and the nodes adjacent to the bronchus intermedius on the right; radiographically, hilar shadow may be created by enlargement of both hilar and interlobar nodes |
| 11. Interlobar nodes | Nodes lying between the lobar bronchi |
| 12. Lobar nodes | Nodes adjacent to the distal lobar bronchi |
| 13. Segmental nodes | Nodes adjacent to the segmental bronchi |
| 14. Subsegmental nodes | Nodes around the subsegmental bronchi |
From Mountain CF, Dresler CM: Regional lymph node classification for lung cancer staging. Chest 11:1718–1723, 1997.
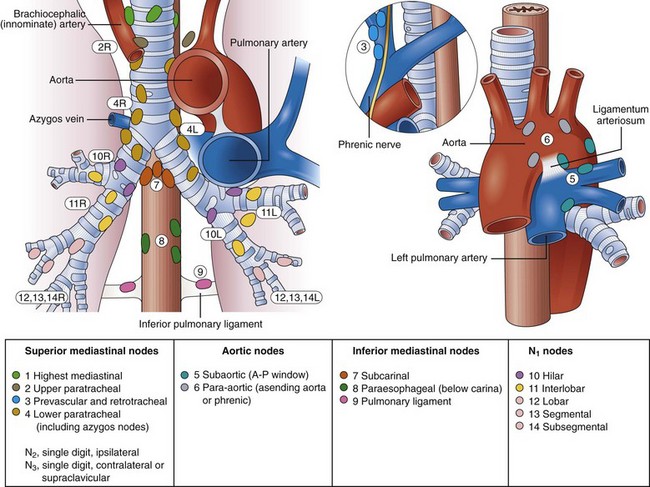
Fig. 31.1 Lymph node stations.
(From Mountain CF, Dresler CM: Regional lymph node stations for lung cancer staging. Chest 111:1718–1723, 1997.)
Various techniques are currently available to diagnose and stage lung cancer, including plain radiography, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), endobronchial ultrasound (EBUS), and EUS. CT scan of the chest is the current standard by which mediastinal lymphadenopathy is detected. Generally, lymph nodes larger than or equal to 1 cm on chest CT scan are considered abnormal. A review of previously published studies reveals an accuracy of CT staging of the mediastinum of 52% to 88%.28–38 This variation has been attributed to the wide range of correlation of lymph node size to the presence of malignant involvement. Although the general trend is increased risk for metastasis correlating with increasing lymph node size, lymph node size is not an accurate criterion for assessing risk. Problems associated with size as a criterion include the inability to differentiate inflammatory or reactive lymph nodes from malignant involvement. In one study, 37% of mediastinal lymph nodes that ranged in size from 2 to 4 cm were benign,38 and 40% of enlarged nodes in another series were not cancerous.39 Similarly, normal-sized lymph nodes can contain foci of cancer. McKenna and colleagues40 found no correlation between the presence of mediastinal nodal metastases and nodal size. Metastases may be found in 21% of normal-sized nodes.41
MRI may be slightly superior to CT in the detection of mediastinal disease,42 and PET has been shown to be superior to CT for staging for the mediastinum.43,44 PET does not rely on an arbitrary cutoff of size to diagnose malignant nodes but detects the increased glycolytic rate in metabolically active tumors. In a meta-analysis, PET had a sensitivity of 79% and a specificity of 91% compared with CT, which had sensitivity and specificity of 60% and 77%, for the detection of mediastinal disease.43 In another meta-analysis by Toloza and colleagues,44 the performance characteristics of CT, PET, and EUS for staging the mediastinum in NSCLC were compared. PET was more accurate than CT or EUS for detecting mediastinal metastases with a sensitivity of 84% and a specificity of 89% for PET compared with CT (sensitivity 57% and specificity 82%) and EUS (sensitivity 78% and specificity 71%). However, PET is limited for small lesions (≤1 cm), has false-negative results in tumors with low metabolic activity, and has false-positive results in benign lesions such as granulomatous disease. Although PET has a relatively high sensitivity, because of the importance and implications of staging, specificity is still too low, and pathologic staging is still generally sought.45–47
Fritscher-Ravens and associates48 performed a prospective comparison of CT, PET, and EUS for the detection of metastatic lymph nodes metastases in patients with lung cancer being considered for operative resection. After bronchoscopic evaluation, CT, PET, and EUS were performed to evaluate potential mediastinal involvement with bronchoscopic biopsy and cytology–proven (n = 25) or radiologically suspected (n = 8) lung cancer before surgery. Surgical histology was used as the “gold standard” and revealed NSCLC in 30 patients, neuroendocrine tumor in 1 patient, and benign disease in 2 patients. With respect to the correct prediction of mediastinal lymph node stage, the sensitivities of CT, PET, and EUS were 57%, 73%, and 94%; specificities were 74%, 83%, and 71%; and accuracies were 67%, 79%, and 82%. Results of PET could be improved when combined with CT (sensitivity 81%, specificity 94%, accuracy 88%). The specificity of EUS (71%) was improved to 100% by fine needle aspiration (FNA) cytology. The authors concluded that no single imaging method alone was conclusive in evaluating potential mediastinal involvement. They also suggested that CT may be necessary to evaluate the pretracheal region and the rest of the thorax and that PET may be valuable to detect distant metastases.
Whenever enlarged lymph nodes are seen in the mediastinum on chest CT scan, standard practice is to perform a lymph node biopsy for more accurate staging. The traditional methods for performing a lymph node biopsy are via CT or bronchoscopy or both. Bronchoscopy with FNA is commonly used to evaluate suspicious paratracheal, hilar, and subcarinal lymph nodes seen on CT.49–52 The role of bronchoscopy in the diagnosis and staging of NSCLC is well established and has a sensitivity of approximately 60%.53–59 Bronchoscopy is unable to access the aortopulmonary window or the inferior mediastinal nodes, however. CT-guided biopsy of the mediastinum is limited by overlying vascular and bony structures. When the lymph node status is not determined with CT or bronchoscopy or both, mediastinoscopy and in some cases limited thoracotomy are performed to clarify the disease stage.37,60–62 However, these procedures are more invasive and require general anesthesia and inpatient recovery, increasing the time, cost, and risk of the staging process.63
Endoscopic Ultrasound
The advent of EUS has made it possible to image the GI tract and surrounding extraluminal structures such as the mediastinum with precise resolution. EUS has played an increasingly important role as an accurate and safe method for staging patients with NSCLC.64–85 With the advent of transesophageal EUS-guided FNA, suspicious posterior mediastinal lymph nodes, including aortopulmonary window, subcarinal, and inferior (below carina) paraesophageal nodes, can be sampled. Tracheal air artifact generally precludes reliable assessment of the anterior mediastinum lesions, pretracheal nodes, and upper paratracheal nodes; however, the advent of EBUS technology seems to be minimizing this limitation. The use of EUS and EBUS technology can enhance the overall accuracy for detecting mediastinal lymph node metastasis.
The results of an early pilot study evaluating the role of EUS in 17 patients with lung cancer found EUS to be very accurate at detecting mediastinal lymphadenopathy with an overall accuracy of 71% versus 41% for CT.83 However, the capability of performing EUS-guided FNA was unavailable during this initial study. Sampling of suspicious lymph is essential except in N1 nodes. In the 1990s, several prospective studies evaluated the accuracy of EUS, EUS-guided FNA, and chest CT scan in detecting and staging mediastinal lymph node metastasis in patients with NSCLC based on correlation with surgical staging.69,70,75 Gress and colleagues75 reported a study consisting of patients with NSCLC and enlarged mediastinal lymph nodes (>1 cm) seen on chest CT scan. EUS-guided FNA was performed for suspicious contralateral posterior mediastinal or subcarinal lymph nodes. EUS criteria used to differentiate benign from malignant lymph nodes resulted in an accuracy of 84% compared with 49% for CT. The sensitivity and specificity of CT was 64% and 35%, which compared with a sensitivity and specificity of 86% and 83% for EUS. The addition of transesophageal EUS–guided FNA improved the overall accuracy of lymph node staging to 96% with a sensitivity of 93% and a specificity of 100%. The combination of CT and EUS did not improve overall accuracy above that for EUS alone for detecting lymph node involvement. However, the addition of CT aided in evaluating the extent of the lung cancer, detecting distant metastasis not seen by EUS and evaluating anterior and pretracheal nodes, which are not imaged by EUS. EUS was best at accurately detecting mediastinal lymph node metastasis in the aortopulmonary window (station 5), subcarinal (station 7), and paraesophageal (station 8) regions (see Fig. 31.1).
These findings are similar to the studies reported by Giovannini and colleagues73 and Silvestri and coworkers,74 who reported sensitivities of 81% and 89% and specificities of 100% each. A recent meta-analysis by Micames and colleagus67 showed a pooled sensitivity of 83% and a pooled specificity of 97% for EUS-FNA staging of NSCLC.
Although still not approaching the “gold standard” of mediastinoscopy and lymph node biopsy, EUS-FNA has greatly improved minimally invasive staging of NSCLC. As noted earlier, where EUS fails is in the anterior mediastinum where tracheal air artifact generally precludes reliable assessment of the anterior mediastinum lesions, pretracheal nodes, and upper paratracheal nodes. In an attempt to view the anterior mediastinum, radial EBUS was initially developed in the 1990s. There was limited use of the technology, however, because it was impossible to perform real-time guided FNA of lesions. In more recent years, convex, linear probe technology has been developed and now allows for real-time EBUS-guided transbronchial needle aspiration (TBNA). A systematic review of EBUS-TBNA showed that it was a safe and effective modality to aid in the staging of NSCLC with a sensitivity ranging from 85% to 100% without reported complications.68 Gu and colleagues69 performed a meta-analysis of studies aimed at measuring EBUS-TBNA accuracy and found a 93% sensitivity and 100% specificity in detecting mediastinal lymph node metastasis. Given the high sensitivity and specificity of both EUS-FNA and EBUS-FNA, many investigators have been seeking to perform a complete, “medical mediastinoscopy,” with the hope of avoiding the more traditional, invasive staging, including mediastinoscopy, of NSCLC before operative intervention.
The largest study to date was performed by Wallace and colleagues,70 who compared the use of TBNA, EUS-FNA, and EBUS-TBNA using pathologic confirmation of malignancy or benign disease as the diagnostic standard. This group found EBUS-FNA to be more sensitive, with a higher negative predictive value than TBNA. More importantly, they found the combination of EBUS-FNA with EUS-FNA to have a sensitivity of 93%, a positive predictive value of 100%, and a negative predictive value of 97%. These results suggest that EBUS-FNA may complement EUS-FNA and possibly could provide near-complete, minimally invasive staging of the mediastinum in patients with NSCLC. These combined modalities may eventually eliminate the need for mediastinoscopy or other invasive surgical procedures for staging purposes.
Endoscopic Ultrasound Technique for Imaging the Mediastinum
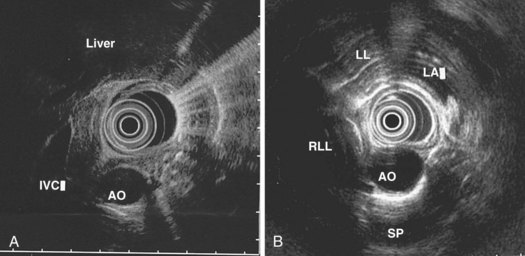
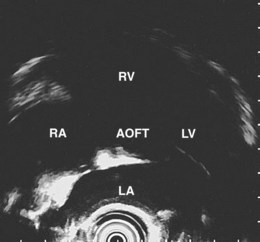
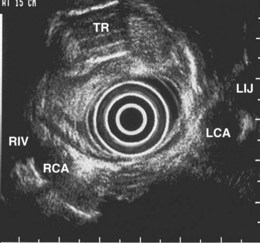
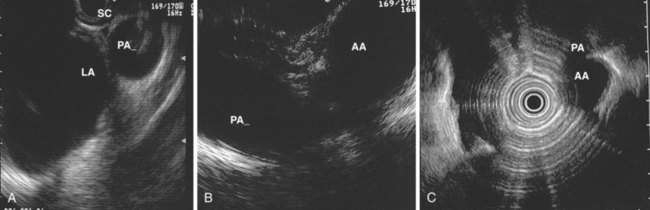
The right lung appears as hyperechoic rings emanating from the 9 o’clock position, whereas the left lung appears at the 2 o’clock position. In the midesophagus, the right and left bronchi are easily demarcated by the hyperechoic rings (echogenic air) seen at the 11 o’clock and 1 o’clock positions. The two bronchi join together to form the trachea normally at 27 to 28 cm from the incisors. The azygos vein can be seen coming into position to the right of the aorta and moves anterior to the spine and toward the right lung. As the endoscope is withdrawn further, the azygos vein can be seen to move forward and extend anteriorly into the superior vena cava. The ascending aorta can be difficult to trace because this structure runs deep to the hilar structures (pulmonary vessels), and because of air within the bronchi and trachea, the ascending aorta is often not fully imaged. In the proximal esophagus, the aortic arch is identified on the left and moves rightward and anteriorly across the screen. In the cervical esophagus, above the level of the aortic arch, the carotid vessels and, occasionally, the thyroid gland can be seen (Figs. 31.2, 31.3, and 31.4).
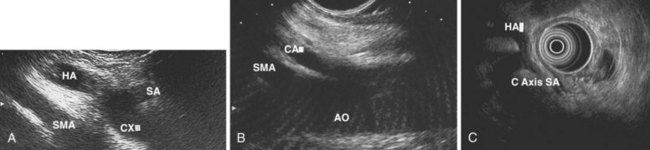
Evaluation of the mediastinum using linear EUS requires rotation of the echoendoscope every few centimeters for a thorough evaluation. As in radial EUS, vascular structures provide the major landmarks for orientation, and the home base structure is the descending aorta, which is first located approximately 35 cm from the incisors. The echoendoscope is rotated initially clockwise (right) bringing structures anterior to the esophagus into view and then counterclockwise (left) bringing posterior structures into view. The left atrium is found by rotating the shaft of the scope 180 degrees in the distal esophagus to midesophagus until a large, echolucent structure is seen within which the mitral valve leaflets are located. By tipping the scope upward and with slight withdrawal, the subcarinal lymph node station is located immediately beneath the endoscope at approximately 27 cm between the left atrium and right pulmonary artery. The aortopulmonary window is located by following the descending aorta cephalad to the arch and pushing the endoscope in again about 2 cm. The endoscope is turned 90 degrees clockwise and tipped up slightly until a cross-sectional view of the aortic arch and the more distally located left pulmonary artery are seen. The area between these structures is known as the “AP window.” Another potentially important area for FNA of lymph nodes is the celiac axis. This area is located by finding the abdominal aorta at the level of the GE junction and the takeoff of the celiac artery with the superior mesenteric artery just distal to this (Figs. 31.5 and 31.8).
Techniques for Staging Non–Small Cell Lung Cancer with Endoscopic Ultrasound
The preparation of the patient is the same as for standard endoscopy. Prophylactic antibiotics are not administered unless recommended by the American Heart Association or the American Society of Gastrointestinal Endoscopy because EUS-guided FNA of mediastinal lesions is not associated with significant bacteremia. However, prophylactic quinolone antibiotics are recommended for FNA of cystic mediastinal lesions, which is similar to the recommendations for pancreatic cystic lesions and perirectal lesions.86–93 After informed consent is obtained and conscious sedation is administered (we have found propofol to be effective), the instrument is advanced into the stomach, and the celiac axis is imaged. The probe is slowly withdrawn to the GE junction and then cephalad using radial scanning images generally obtained with 7.5-MHz frequencies at each 1-cm interval while keeping the aorta at the 5 o’clock or 6 o’clock position. All mediastinal lymph nodes seen are “mapped” by location according to the American Thoracic Society classification scheme (see Box 31.1, Table 31.1, Fig. 31.1, and video on expertconsult.com).12–14
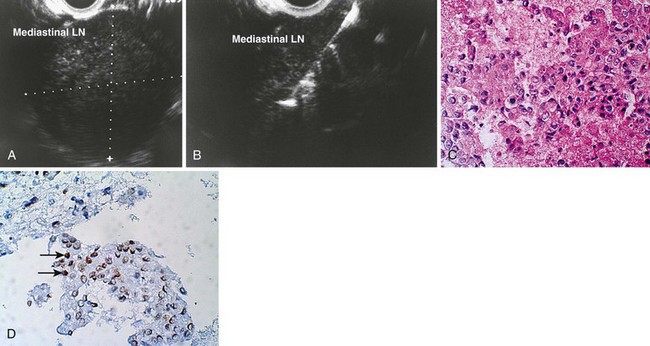
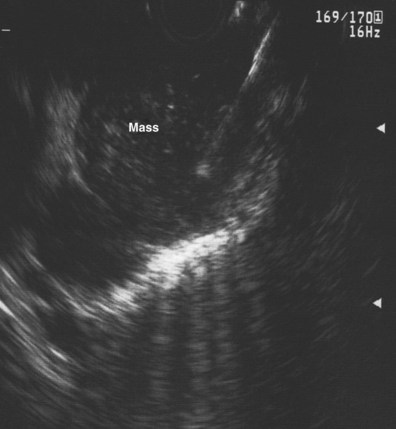
An objective determination is made as to whether the mediastinal lymphadenopathy detected by EUS is consistent with benign or malignant status according to previously reported studies using the same criteria.82,87,94–100 EUS criteria used to diagnose malignant lymph nodes are round shape, sharp distinct borders, hypoechoic texture, and a short-axis diameter greater than 5 mm. Each of these parameters should be present for a lymph node to be considered as potentially malignant; however, FNA has significantly improved the sensitivity and specificity of detection of malignant lymph nodes.73–75,84,85,87,100,101
Technique for Performing Endoscopic Ultrasound–Guided Fine Needle Aspiration
Mediastinal Lymph Nodes
The EUS-guided FNA biopsy technique was initially developed for use with the linear array instrument and has been described elsewhere.95–97,102,103 The unique viewing angle of the linear array transducer allows for observation of the needle as it exits the biopsy channel and enables direction of the needle tip into the target lesion. A similar technique using a radial scanning echoendoscope has been reported; however, serious complications have been described via this technique, and it is not recommended.94,103
EUS-guided FNA involves the insertion of the FNA catheter device through the accessory channel of the echoendoscope followed by deployment of the needle under EUS guidance into the lymph node to be sampled. The handle mechanism is secured to the accessory port, and if the instrument has an elevator, the elevator should be fully released into the down position to allow easy passage of the needle. The elevator can be used during the biopsy to direct the needle gently into the lesion. Doppler is used to identify surrounding vascular structures. The FNA needle is slowly advanced toward the target lesion. With certain needles, it helps if the stylet is withdrawn a few millimeters (2 to 3 mm), and the needle and the stylet are then directed into the target. When the needle has entered the lesion, the stylet is advanced (to clear the needle) and then removed. The endosonographer or assistant applies suction to the catheter system using a 5-mL or 10-mL Luer-Lok syringe. Suction is followed by “in-and-out” movements of the catheter after firmly locking the needle-catheter system to the appropriate depth so that the needle is not advanced beyond a desired depth. Typically, we make 7 to 10 gradual in-and-out movements within the lesion. Before removing the needle, the negative pressure is released slowly, the needle is removed from the lesion, and subsequently the needle system is unscrewed from the echoendoscope. It has been suggested to perform EUS-guided FNA of lymph nodes without the use of suction because suction may result in a bloody sample that may be more difficult for the cytopathologist to examine.100
Preliminary cytology findings are obtained immediately during the FNA procedure by a cytopathologist or cytotechnologist present during the study. We recommend having a cytopathologist or cytotechnologist present during the EUS-guided FNA portion of the procedure because it can improve the efficiency of the technique. If a cytopathologist or cytotechnologist is unavailable, two to three passes should be taken by the endosonographer for lymph nodes (or liver metastases) and five to six passes should be taken for masses (similar to pancreatic masses) to ensure adequate cellularity in more than 90% of cases.100,104 However, this approach is associated with a 10% reduction in definitive cytologic diagnoses, increased time and risk, and potentially the need for additional needles.104
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree