Chapter 53 Etiology, pathogenesis, and diagnostic assessment of acute pancreatitis
Overview
The incidence of acute pancreatitis ranges from 10 to 46 cases a year per 100,000. For 2004, per 100,000 population, 94 cases of pancreatitis were listed as the hospital discharge diagnosis, suggesting an increasing incidence in the United States. Pancreatitis was the eleventh most common cause of death from digestive diseases and the fifth most common nonmalignant cause for mortality, just after peptic ulcer disease (Everhart & Ruhl, 2009). The mortality rate of acute edematous interstitial (mild) pancreatitis is less than 1%, whereas in patients with hemorrhagic necrotizing (severe) pancreatitis, it is reported to be between 10% and 24% (see Chapter 52). Clinically acute pancreatitis thus affects a heterogeneous group of patients in whom determining the etiology, severity assessment at admission, and triage to either symptomatic or intensive care treatment remains a significant challenge.
Etiology and Pathogenesis of Acute Pancreatitis
Pancreatitis is an inflammatory disorder of the exocrine pancreas caused, in most cases, by immoderate alcohol consumption or the passage of gallstones (see Chapters 30 and 35). Recent studies involving animal and isolated cell models have elucidated many of the pathophysiologic, cellular, and molecular processes involved in the disease onset. More than 100 years ago, it was proposed that pancreatitis was essentially a disease in which the pancreas falls prey to its own, prematurely activated digestive enzymes regardless of the underlying etiology (Chiari, 1896).
Etiology and Pathogenesis of Acute Biliary Pancreatitis
In roughly 30% of acute pancreatitis cases, gallstone disease is the underlying cause. The prevalence of gallstones is 18.8% (Volzke et al, 2005), which puts nearly one fifth of the population at risk of developing an acute episode of biliary pancreatitis at some point in their lifetime. Although a male/female ratio of 1 : 3 exists for gallstone disease, acute biliary pancreatitis is observed with a ratio of 1 : 1.4 to 1 : 1.7, suggesting that men with gallstones are at a greater risk of developing biliary pancreatitis than women with gallstones. Further studies have shown that the gender ratio changes with age. Imrie and colleagues reported a male/female ratio of 1 : 2.7 in younger patients with gallstone pancreatitis, whereas the ratio changed to 1 : 1.1 in patients older than 50 years. In the group between 60 and 70 years of age, the gender predominance was reversed; more men than women had biliary pancreatitis (Imrie & Blumgart, 1975; Imrie & Whyte, 1975).
Ever since 1856, when Claude Bernard reported that bile injection into the pancreatic duct of laboratory animals leads to acute pancreatitis, the pathophysiology of gallstone pancreatitis has been a matter of dispute (Bernard, 1856; Lerch & Aghdassi, 2009). The first investigator to systematically address the issue of biliary pancreatitis was Eugene Lindsay Opie, who in 1901 published two autopsy reports, from which he concluded that two mutually exclusive triggering mechanisms exist for gallstone-induced pancreatitis (Opie, 1901a, 1901b). He tried to support his hypotheses with a series of animal studies. The first autopsy report showed that an impacted gallstone had occluded the orifice of the pancreatic duct, and the patient had died from acute pancreatitis (Opie, 1901a). When Opie simulated this finding by pancreatic duct ligation in cats, he noted the development of pancreatic tissue and fat necrosis and proposed pancreatic outflow obstruction as the triggering event for acute pancreatitis. Unfortunately, his first “impaired outflow hypothesis” was rapidly forgotten after he published his second hypothesis.
In another patient who underwent a postmortem examination, Opie found a distinctly different anatomic situation, which he regarded to be of pathophysiologic relevance. The impacted stone at the papilla of Vater had created a communication between the common bile duct and the main pancreatic duct that would have permitted the patient’s bile to enter the pancreatic duct. Opie proposed the presence of infected bile in the pancreatic duct as the triggering mechanism of pancreatitis, the so-called common channel hypothesis. We and others have tested Opie’s common channel hypothesis in the past, using the opossum model of acute necrotizing pancreatitis. This model appears ideally suited to test whether bile reflux into the pancreatic duct or blockage of pancreatic secretion triggers pancreatitis because the opossum not only possesses a gallbladder, a common bile duct, and a single pancreatic duct but also has a long communication between the two ductal systems. If this common channel is ligated at the papilla of Vater, it creates a communication between the pancreatic and bile ducts, through which bile could potentially flow (Lerch et al, 1992).
Our experiments consistently showed that neither a common channel nor reflux of bile into the pancreas is required for the onset of acute necrotizing pancreatitis (Lerch et al, 1993), but pancreatic duct ligation is sufficient for triggering the disease. For obvious reasons, no controlled experiments in humans that would replicate the opossum data are possible; however, support comes from case observations in which unique anatomic situations allow for pathophysiologic interpretations (Lerch et al, 1994a, 1994b; Pohle et al, 2003). One of these cases is that of a young woman in whom an impacted gallstone at the papilla had caused acute pancreatitis, and a surgically inserted common bile duct T-tube had prevented any potential bile reflux into the pancreas (Lerch et al, 1994a, 1994b; Pohle et al, 2003). This case demonstrates further that therapeutic measures aimed at preventing bile reflux through a common channel will not afford protection against pancreatitis, but those aimed at preventing pancreatic duct obstruction will (Lerch et al, 1994a).
Patients in whom congenital biliopancreatic fistulas have caused a life-long flow of bile through the pancreatic duct without ever causing pancreatitis provide more arguments against Opie’s common channel theory (Lerch et al, 1994b; Pohle et al, 2003). Although these studies and observations firmly put the blame for gallstone pancreatitis on the mechanisms that involve impairment of pancreatic outflow, rather than bile reflux into the pancreatic duct, there may still be a role for cholestasis in regulating its severity. Senninger and colleagues (1996) found in the opossum model that bile duct obstruction in addition to pancreatic duct ligation can aggravate pancreatitis, and two groups found independently that increased bile acid concentrations, such as those in cholestasis, can increase the susceptibility of pancreatic cells to injury (Kim et al, 2002; Perides et al, 2009; Voronina et al, 2002, 2004, 2005).
Experimental studies and reports from human case series have tried to elucidate the mechanisms through which migrating gallstones cause pancreatitis. In spite of its former popularity, Opie’s common channel hypothesis of bile reflux into the pancreatic duct appears no longer valid; the duodenal content reflux hypothesis has also been firmly refuted in human studies (Hernandez & Lerch, 1993). The most accurate description of the pathophysiology of gallstone pancreatitis is based in Opie’s original report, in which he proposes pancreatic outflow obstruction as the most critical event for the disease onset. To what extent cholestasis and circulating bile acids contribute to acinar cell injury in humans and what factors determine the ultimate disease severity must be elucidated by future investigations.
Etiology and Pathogenesis of Acute Alcoholic Pancreatitis
Alcohol abuse is a major cause of acute pancreatitis and the leading cause of chronic pancreatitis. Although the incidence of pancreatic disease increases as a function of the extent of alcohol abuse in a population, only a minority of subjects who greatly abuse alcohol develop pancreatitis. According to studies from Marseille (Levy et al, 1995), the logarithm of the relative risk of pancreatitis increases linearly as a function of the quantity of alcohol and protein consumed. Unlike the liver, no alcohol toxicity threshold has been established beyond which the pancreas is damaged. Furthermore, the type of alcoholic beverages consumed appears to be less relevant. Patients with pancreatitis and alcohol-induced liver cirrhosis do not generally differ with regard to their daily intake of alcohol. However, the duration of alcohol consumption is shorter in pancreatitis. In most studies the time between the onset of alcohol abuse and first pancreatitis symptoms ranges from 7 to 29 years. The prevalence of pancreatitis clearly correlates with the alcohol consumption in a given population (Ammann & Muellhaupt, 1994).
The mechanisms involved in alcohol-induced pancreatitis have been extensively studied in ethanol-fed laboratory animals. Although a number of biologic cell changes have been reported from these studies, their relevance to the human disease is questionable, because neither rats nor mice develop pancreatitis when fed a high-alcohol diet over an extended time. Animal studies have led to the working hypothesis that alcohol abuse sensitizes individuals to pancreatitis but does not directly cause the disease. Experimentally, ethanol can mediate its damaging effect on pancreatic acinar cells through several mechanisms. First, ethanol affects the inflammatory signaling cascade in pancreatic acini, namely the NF-κB pathway (Tando et al, 1999). Furthermore, ethanol feeding results in a decrease of in the expression and/or activity of both “initiator” and “executioner” caspases, leading to cell death (Fortunato et al, 2006).
Recent studies observing the effect of ethanol on the intracellular activation of digestive protease zymogen were rather successful in elucidating potentially disease-relevant mechanisms. Ethanol feeding enhanced the expression and activity of cathepsin B, which catalyzes 80% of the intracellular trypsinogen activation and results in active trypsin and, ultimately, cell necrosis (Fortunato et al, 2006; Halangk et al, 2000).
A number of studies found that ethanol can sensitize acinar cells to cholecystokinin (CCK)-induced procarboxypeptidase A1 processing in vitro, and it can also sensitize the pancreas to various forms of pancreatitis in vivo (Katz et al, 1996; Pandol et al, 1999; Saluja et al, 1997). Thus ethanol is believed to enhance the pathophysiologic stimuli that induce pancreatitis, and this process may explain the effects of ethanol toxicity on the pancreas.
A recent study addressed several issues relevant to zymogen activation and the effects of ethanol in isolated pancreatic acini (Gorelick, 2003). Trypsinogen and chymotrypsinogen were observed to exhibit distinct patterns of activation in response to supraphysiologic concentrations of the CCK-analogue cerulein, supraphysiologic concentrations of which are known to cause calcium-dependent intracellular protease activation and cell death; this model is regarded as in vitro pancreatitis. Moreover, ethanol and other alcohols were shown to sensitize acinar cells to cerulein-induced trypsin and chymotrypsin activation, and other short-chain n-aliphatic alcohols—methanol, propanol, and butanol—enhanced the effects of cerulein on the acinar cell. Ethanol alone, on the other hand, was not found to induce pancreatitis or zymogen activation in experimental models of pancreatitis (Lu et al, 2002; Ramo, 1987).
The aforementioned descriptions of the effects of alcohol on the pancreas do not take into consideration the unique metabolism of ethanol in the pancreas. The pancreas differs from the liver in that it transiently converts ethanol to fatty acid ethyl esters (FAEEs) (Gukovskaya et al, 2002; Werner et al, 1997). Recent data suggest that FAEEs cause sustained increases in the cytosolic calcium concentrations, which are closely connected to the premature activation of zymogens, as well as mitochondrial injury, subsequently leading to necrotic cell death (Ponnappa et al, 1997).
In summary, ethanol and its metabolites have multiple effects on the pancreas that are involved in sensitizing the pancreas to pathologic stimuli. These include effects on inflammatory and cell death signaling pathway that can promote inflammation and necrosis. Mainly identified in animal models, the findings are consistent with a recent report that indicates alcohol abuse in humans is a risk factor for pancreatic necrosis during pancreatitis (Papachristou et al, 2006).
Etiology and Pathogenesis of Nonalcoholic and Nonbiliary Pancreatitis
Hyperlipidemia
As with other forms of acute pancreatitis, acute hyperlipidemic pancreatitis presents clinically with varying degrees of severity. Complications typical of acute pancreatitis, such as infected necrosis and pseudocyst formation, also occur in pancreatitis triggered by hypertriglyceridemia (Toskes, 1990). According to Fortson and colleagues (1995), patients coming to medical attention with hyperlipidemic pancreatitis fit one of the following clinical scenarios: they are either patients whose diabetes mellitus is out of control; alcoholics with a lactescent serum; nondiabetic, nonalcoholic, nonobese patients with hypertriglyceridemia caused by nutrition or medication; or patients with familial hypertriglyceridemia.
The diagnosis of acute hyperlipidemic pancreatitis is made as for other etiologies of this disease. However, a few important peculiarities must be considered, such as a lipemic serum that signals hyperlipemic pancreatitis. It is also striking that in more than 50% of patients, serum and urinary amylase levels remain within the normal range (Lesser & Warshaw, 1975). The reason for this phenomenon has long been suspected to be an interference of the test assay with the plasma lipids or an unknown amylase inhibitor in plasma and urine that impairs the assay. This inhibitor has not been identified so far (Fallat et al, 1973; Warshaw et al, 1975). Although during the course of hyperlipemic pancreatitis, serum amylase levels may remain normal, renal amylase clearance increases. In the past, a higher ratio in amylase/creatinine clearance in the urine has been shown to be a diagnostic parameter of hyperlipemic pancreatitis (Fallat et al, 1973; Warshaw et al, 1975); however, this method has not entered clinical routine. Triglycerides are usually above 1000 mg/dL, and when enteral nutrition regimens cannot lower triglyceride levels below that level within 2 days, sometimes even extracorporeal lipid-lowering therapies should be considered (Iskandar & Olive, 2004).
Drug-Induced Pancreatitis
A database of the World Health Organization (WHO) lists 525 drugs that can, as an adverse reaction, induce acute pancreatitis (Table 53.1). Compared with other causes, drugs represent a relatively rare cause of pancreatitis. They should be regarded as a triggering event in patients with no other identifiable cause of the disease who take medications that have been shown to induce pancreatitis. The prevalence of drug-induced pancreatitis is still unclear because most incidences have been documented only as isolated case reports. The overall incidence probably ranges somewhere between 0.1% and 2% of pancreatitis cases.
Table 53.1 Drugs with a Probable or Definite Association to Pancreatitis as Reported in Case Report Summaries
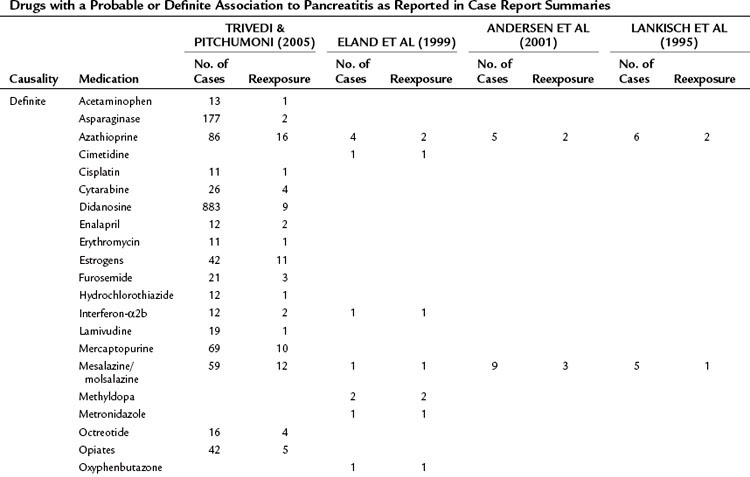
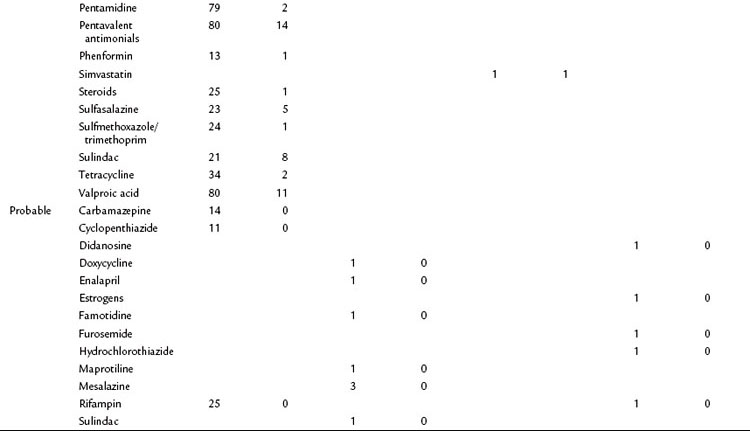
For only very few substances, evidence from controlled trials has been obtained. Epidemiologic data suggest that the risk of pancreatitis is highest for mesalazine (hazard ratio [HR], 3.5), azathioprine (HR, 2.5), and simvastatine (HR, 1.8). Even when a definite association has been demonstrated, it is often impossible to determine whether the drug or the underlying condition for which the drug was taken has conferred the risk of pancreatitis (e.g., azathioprine and Crohn disease or pentamidine and HIV). Knowledge about the underlying pathophysiologic mechanisms and evidence for a direct causality often remains sparse. A definite causality has been established for only 31 drugs, although a cause-effect relationship is generally accepted when symptoms recur upon rechallenge. Available data from case control studies suggest that even drugs with solid evidence for an association with pancreatitis only rarely cause the disease. Even when pancreatitis is induced as an adverse drug event, the disease course is usually mild or even subclinical (Nitsche et al, 2010).
Infectious Causes
A number of viral, bacterial, parasitic, and fungal infections have been linked to acute pancreatitis (Parenti et al, 1996). As early as 1817, infection with mumps has been suspected as a cause of pancreatitis; in 1905, Lemoine described a patient whose symptoms were those typical of mumps who also had acute pancreatitis. Today, acute pancreatitis as part of an infectious disease has to be distinguished from an infectious complication of acute pancreatitis that occurs regardless of the underlying etiology. The incidence of acute pancreatitis in connection with infectious diseases is difficult to determine, as there are hardly any prospective studies, but an incidence of less than 2% is generally assumed. In many cases, somewhat increased amylase and lipase levels are described in the course of an infectious disease, and the patient is often prematurely diagnosed with pancreatitis; however, often no other evidence for pancreatitis is present, either clinically or on imaging studies (Parenti et al, 1996).
The most frequent association of a viral infection with acute pancreatitis is mumps. The incidence of pancreatitis associated with mumps (parvovirus B19) is 0.3% to 14% according to the relevant literature (Kaplan et al, 1988; Witte & Schanzer, 1968). Acute pancreatitis mainly occurs after the swelling of the parotid gland has subsided, usually 8 to 14 days after the initial onset of the disease. Every now and then, acute pancreatitis occurs up to 1 week prior to parotitis. In rare individual cases, acute pancreatitis has been reported as the only manifestation of a mumps infection (Kaplan et al, 1988; Witte & Schanzer, 1968). Acute pancreatitis associated with mumps is usually mild, with symptoms that normally persist only 3 to 7 days. For a laboratory-based diagnosis of acute pancreatitis, lipase activity should be measured. Amylase measurements may result in an incorrect diagnosis because increased activity may be due to amylase from affected salivary glands and may not necessarily reflect acute pancreatitis. There is no specific therapy for either mumps infection or for acute pancreatitis associated with mumps (Kaplan et al, 1988; Witte & Schanzer, 1968).
The second most common viral infection associated with pancreatitis is coxsackievirus B. During a coxsackievirus type B5 aseptic meningitis epidemic in Japan, 31% of patients showed an increase in amylase activity in serum or urine (Nakao et al, 1964). During an epidemic of coxsackievirus type B4 infection in Australia, acute pancreatitis could be confirmed in 3% of the patients (Murphy & Simmul, 1964). In prospective and retrospective serologic studies, coxsackie virus infections were found in 0% to 11% of patients with positive titers for acute pancreatitis (Parenti et al, 1996). In mouse experiments, acute pancreatitis has successfully been induced with coxsackie viruses; a direct infection of acinar and islet cells with the virus led to an inflammatory reaction and necrosis.
An acute infection with hepatitis virus A, B, or C may result in a secondary infection of the pancreas (Achord, 1968). When a mild form of acute hepatitis occurs, an increase in amylase activity can be detected in up to 30% of patients. When the course of acute hepatitis was severe and ultimately lethal, acute pancreatitis was confirmed on autopsy in up to 44% of patients.
Autopsy studies have detected pathologic changes of the pancreas in up to 50% of patients who died from AIDS. Similarly, 50% of HIV patients showed elevated amylase levels and clinical signs of acute pancreatitis (Cappell & Hassan, 1993; Pezzilli et al, 1992). However, a number of factors could have caused hyperamylasemia and acute pancreatitis in patients with HIV. Apart from damage caused by the virus itself, a nonspecific amylase elevation as a result of renal insufficiency or amylase elevations may be caused by extrapancreatic factors, such as damage to the salivary glands; an infection of the pancreas through opportunistic infections; a medication-induced etiology, especially didanosine (ddI); or a pancreatic neoplasm (Cappell & Hassan, 1993).
Individual case reports have been published on acute pancreatitis in association with infections with Epstein-Barr virus, rubella, adenovirus, rubeola, herpes simplex virus, rotavirus, and after mumps vaccinations (Parenti et al, 1996).
Several bacterial infections have been found in association with acute pancreatitis, such as Yersinia enterocolitica and Y. pseudotuberculosis, Salmonella enteritis and S. typhimurium, Campylobacter jejuni, and Mycoplasma pneumoniae. Of those with yersiniosis, 2% to 14% were diagnosed with secondary acute pancreatitis. In these patients, serotypes 3 and 9 (Y. enterocolitica) and IA (Y. pseudotuberculosis) were isolated. The majority of these patients also had gastroenteritis, and the course of pancreatitis was mild in all cases (Leino et al, 1987; Saebo & Lassen, 1991). Elevated serum amylase and lipase levels in serum were diagnosed in 43% of the patients with confirmed S. typhimurium and in 71% of the patients with S. enteritidis infection. In approximately half of these patients, signs of acute pancreatitis were detected by ultrasound examination (Hermans et al, 1991). Acute pancreatitis induced by a Campylobacter infection is a rare event, with reports on approximately 20 cases in the literature. Amylase and lipase levels elevated three to six times above normal were reported, but the course of the acute pancreatitis was again mild in all cases (Hermans et al, 1991).
Parasites are known to be a relevant cause of acute pancreatitis, mainly on the Indian, African, and Asian continents (see Chapter 45). The incidence of ascariasis differs from region to region worldwide but is the most common type of helminthic infection in humans. An endemic manifestation is found mainly in tropical and subtropical countries. In India, for example, ascariasis is the second most common cause of acute pancreatitis, next to gallstone pathogenesis (Parenti et al, 1996). The worms can travel from the intestines to either the biliary or pancreatic ducts, where they lead to obstruction. The obstruction of the flow from the pancreatic duct then triggers pancreatitis as proposed by Opie’s first hypothesis. In addition to examining the stool for worm eggs, the disease can be diagnosed by sonography or endoscopic retrograde cholangiopancreatography (ERCP). The appropriate treatment is a combination of standard therapy for acute pancreatitis and antihelminthic therapy (Lim & Ko, 1990).
The Chinese liver fluke Clonorchis sinensis can lodge in the biliary tract and often does not cause symptoms for years (see Chapter 45). The endemic form of the disease is found mainly in Asia (Lim & Ko, 1990; Parenti et al, 1996). An obstruction of the biliary or pancreatic ducts is rarely caused by the presence of the worm alone. Via adenomatous proliferation and squamous metaplasia, infection by C. sinensis causes periductal fibrosis, which then leads to further obstruction that can result in acute pancreatitis.









