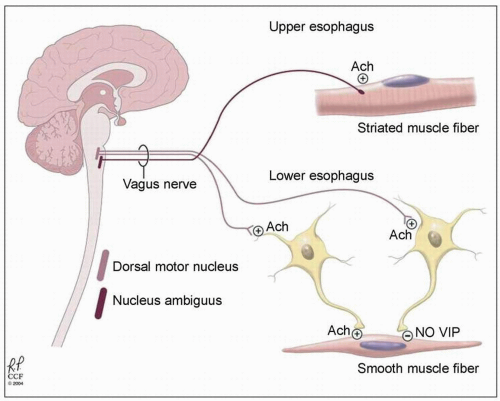
plexus has two types of post-ganglionic neurons: (i) excitatory cholinergic neurons; and (ii) inhibitory nitrinergic (nitric oxide, NO) neurons and vasoactive intestinal polypeptide- (VIP) containing neurons.
‘Bird’s beak’ narrowing of the LES with incomplete opening.
Loss of primary peristalsis.
Delayed esophageal emptying.
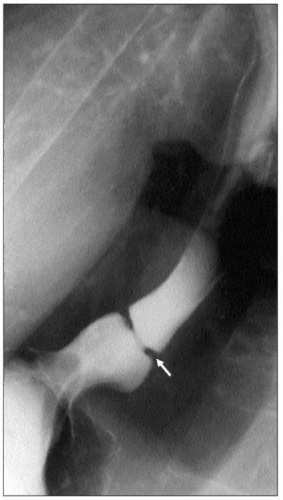
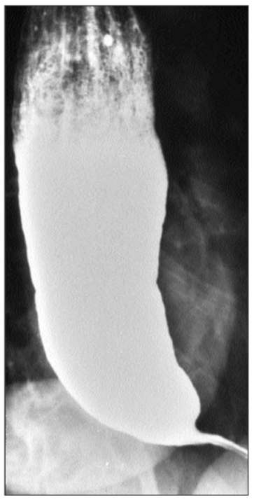 4.3 Characteristic barium esophagram of achalasia. There is poor emptying of the barium from the esophagus which is dilated. There is a characteristic ‘bird’s beak’ narrowing of the distal esophagus due to a non-relaxing LES. |
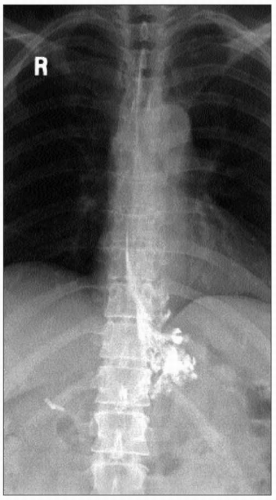
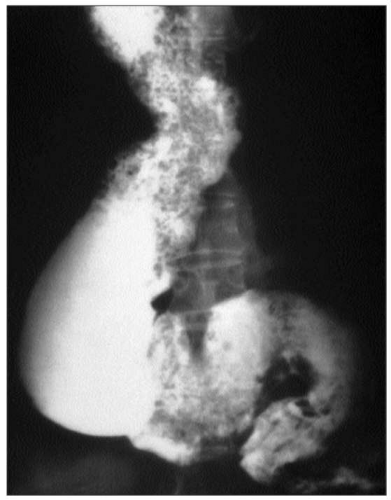 4.4 End-stage achalasia (sigmoid esophagus). The esophagus is markedly dilated and tortuous, forming a sigmoid shape. |
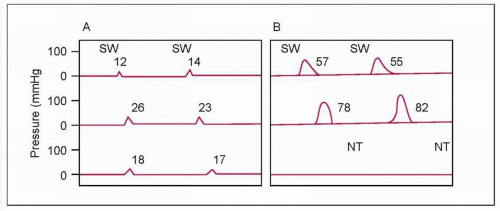
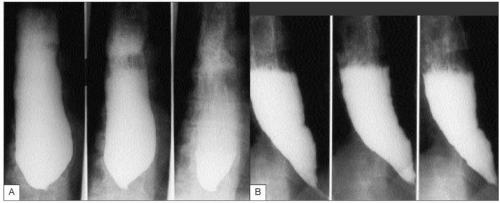 4.5 Timed barium swallow (TBS) in two patients (A and B) with significant delay in esophageal emptying. TBS provides objective evidence of esophageal function and is most useful in diagnosing achalasia and assessing the response to therapies such as pneumatic dilation, or surgical myotomy. It is performed with 250 ml of barium. After the barium is ingested, an X-ray is taken in upright position at time 1, 2, and 5 minutes. The barium height and width at 1, 2, and 5 minutes are measured. In normal population, the barium completely empties in 1 minute. |
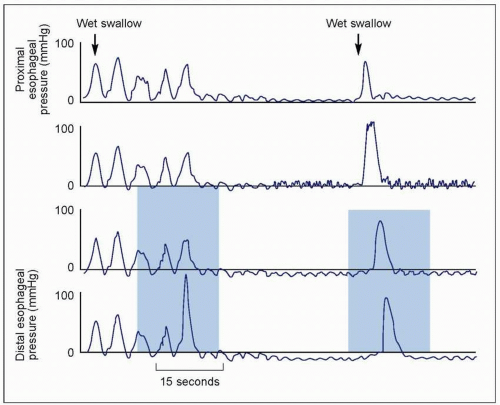
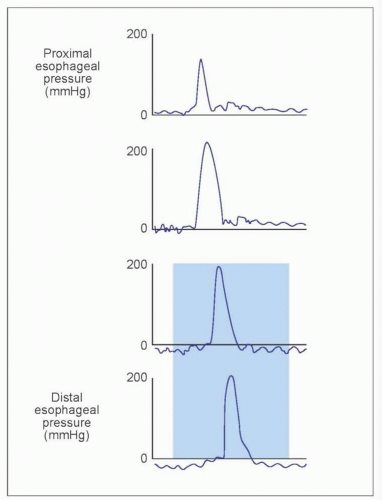
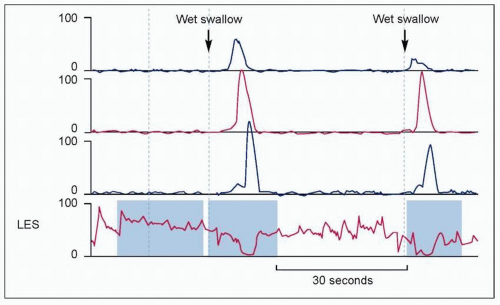
Aperistalsis in the distal two-thirds of the esophagus (4.6).
Simultaneous onset.
Isobaric (identical pressure tracings in all leads).
Abnormal LES relaxation with swallows.
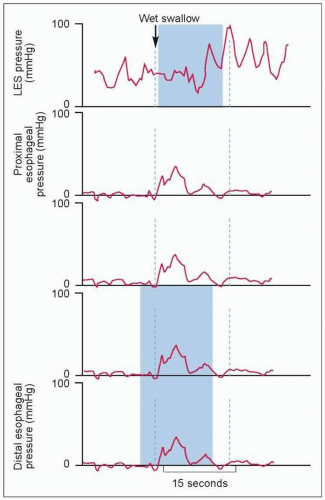 4.6 Manometric findings in achalasia. Achalasia is defined manometrically by aperistalsis and failure of LES relaxation. The pressure tracing shows low amplitude simultaneous contractions. Pressure tracings in all leads are identical or isobaric. Isobaric contractions are due to the esophagus being a closed chamber (dilated esophagus closed by sphincters on both ends), where pressure changes are detected by all manometric sites. In addition, LES does not relax with wet swallow. |
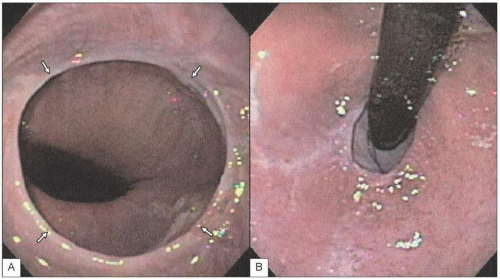
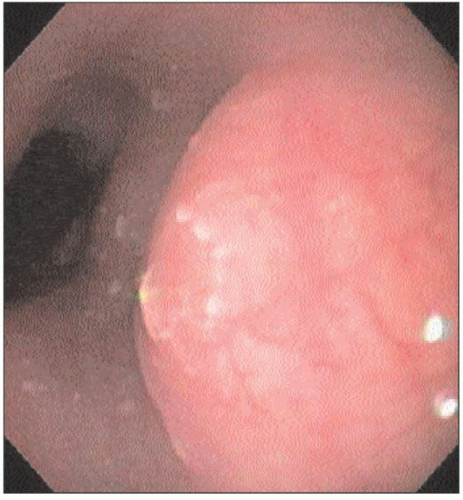
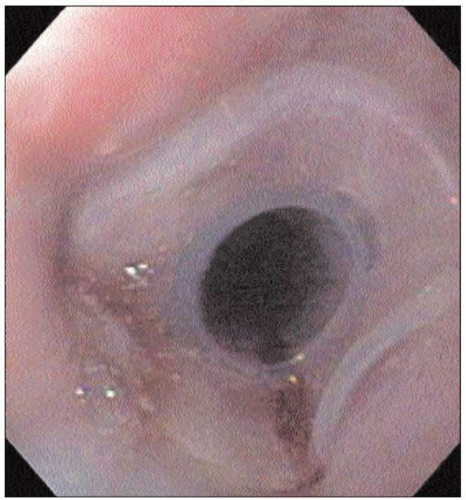
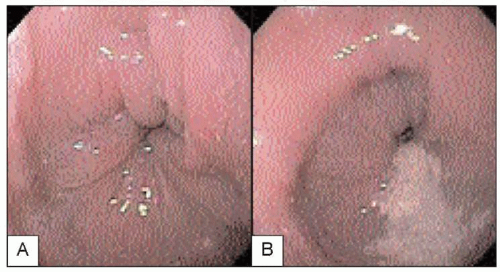 4.7 Upper endoscopy of an achalasia patient reveals a characteristic puckered GEJ. Retained secretions and food in the esophagus are also frequently seen. |
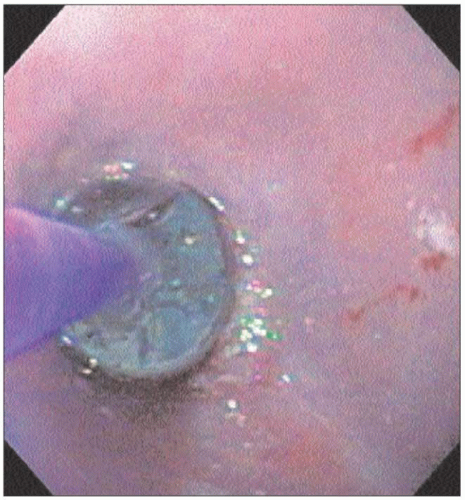
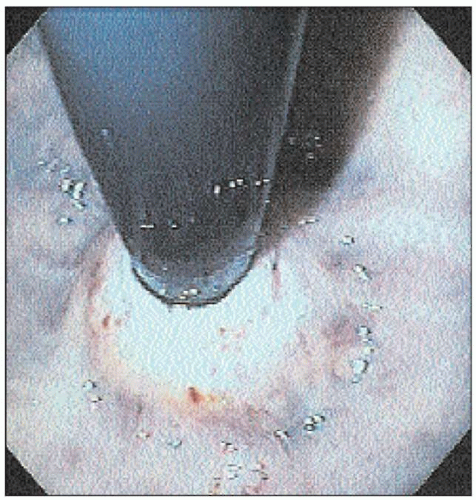 4.8 Retroflexed view of the gastric cardia shows very tight LES around the endoscope. There is no evidence of malignancy causing pseudoachalasia. |
Duration of symptoms <6 months.
Onset after age 60 years.
Excessive weight loss.
Computed tomography (CT) scan showing marked (>1 cm) and/or asymmetric esophageal wall thickening.
4.1 Disorders with manometric and radiologic features similar to achalasia | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
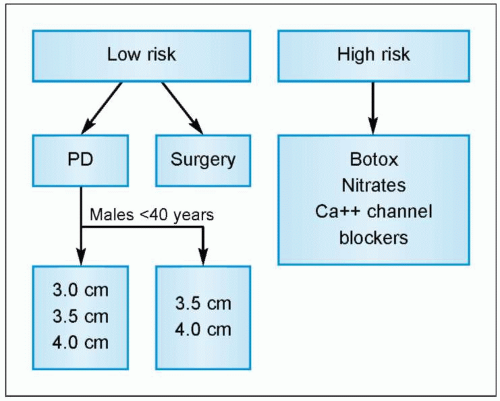
Pneumatic dilation.
Surgical myotomy (Heller).
Botulinum toxin injection.
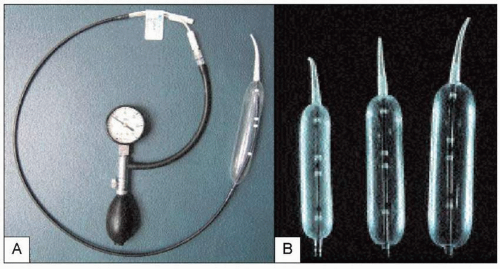 4.10 A: Rigiflex pneumatic dilator. B: The dilator comes in three balloon sizes: 3.0, 3.5, and 4.0 cm. Pneumatic dilation uses air pressure to disrupt traumatically the circular muscle layer of the LES. A 3.0 cm balloon is usually used for initial dilation. With symptom recurrence, repeat dilations are performed in a stepwise graded fashion using larger sized balloons. |
4.2 Comparison of treatments for idiopathic achalasia | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
symptom recurrence, repeat dilations are performed in a stepwise graded fashion using larger sized balloons. The balloon is positioned over a guidewire using either endoscopic or fluoroscopic control across the LES. The balloon is then inflated until the balloon waist (formed by the LES) is obliterated. The pressure applied is usually 10-14 psi (4.11, 4.12). After dilation therapy, all patients undergo gastrograffin esophagram followed by barium esophagram to rule out perforation (4.13).
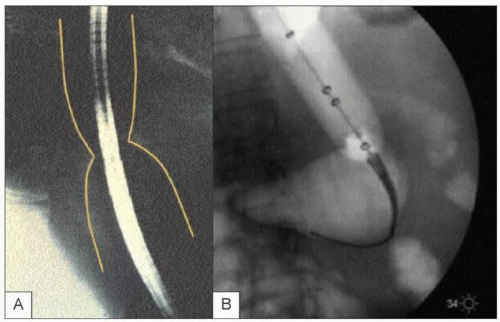 4.12 Pneumatic dilation is usually performed using fluoroscopic control. When the balloon is inflated, a waist is formed secondary to a poorly relaxing LES. The balloon is slowly inflated further until the waist is obliterated. |
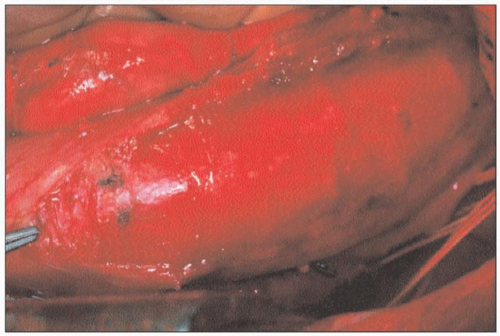 4.14 Open Heller myotomy. Heller myotomy consists of anterior myotomy across the LES. The circular muscle fibers are divided down to the level of mucosa. The myotomy extends to several centimeters above LES and <1 cm onto the stomach. Anti-reflux surgery (Dor fundoplication) is usually performed concomitantly. |
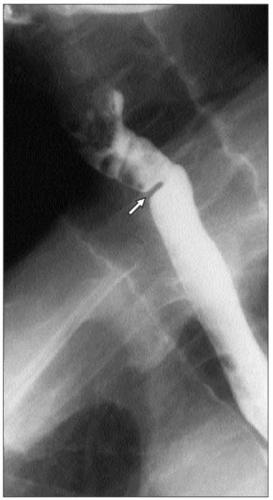
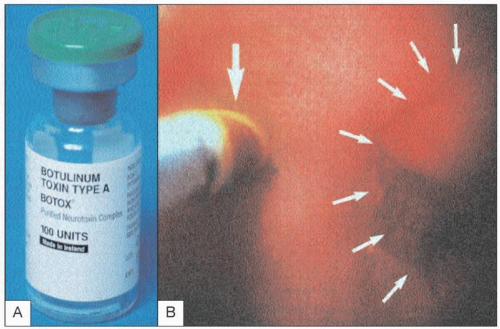 4.15 A: Botulinum toxin injection is performed on patients who are high risk for pneumatic dilation or surgical myotomy, such as the elderly or those with other co-morbidities. Botulinum toxin inhibits acetylcholine release from nerve terminals, thereby blocking the excitatory effects of the cholinergic neurons. B: It is injected at about 1 cm above the gastroesophageal junction (large arrow). The LES is highlighted by the smaller arrows at the puckered GEJ. Botulinum toxin is initially effective in about 85% of patients. However, the response only lasts about 6 months with >50% symptom recurrence in 6 months. |
Barium esophagram is sensitive and specific in detecting motility disorder when carefully performed with videofluoroscopy.
EGD has little role in the evaluation of esophageal dysmotility. It is used in conjunction with barium studies to rule out structural lesions or esophagitis.
Manometry is necessary in order to characterize and define esophageal motility disorder.
4.3 Manometric diagnosis of motility disorders | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
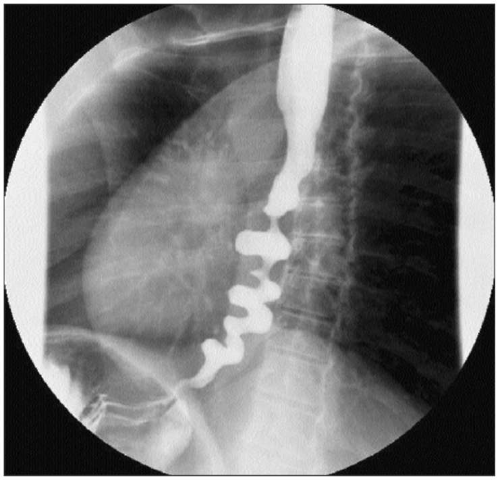 4.17 Barium esophagram in DES. The barium swallow study shows characteristic ‘corkscrew’ appearance. |
Table 4.4 Therapeutic options for esophageal motility disorders | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Table 4.5 Webs and rings | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
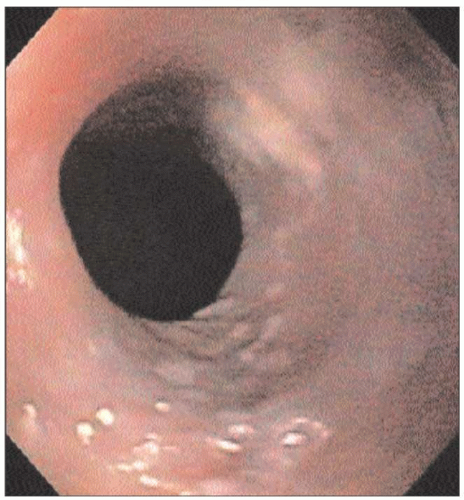 4.22 Endoscopic appearance of a proximal esophageal web. The web appears to be a thin, eccentric lesion. The mucosa is normal in appearance. If located proximally, it is possible to fracture the web during passage through the UES without being aware of its presence. |
common cause of intermittent solid food dysphagia, which may be slowly progressive over years. Other clinical presentations include food impaction and, rarely, perforation.
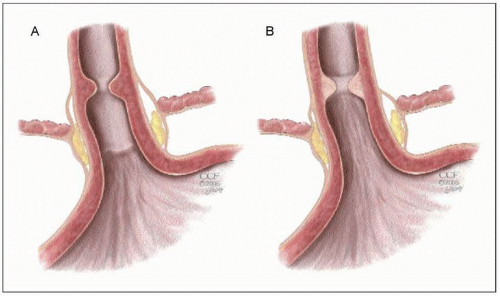 4.25 Schematic figure to show the difference in appearance of esophageal ‘A’ rings (A) and ‘B’ rings (Schatzki’s ring) (B). The ‘A’ ring is located proximal to the SCJ, and is an annular ring composed of hypertrophied muscle. The ‘B’ ring, or Schatzki ring, is located at the SCJ, and is always located in association with a hiatal hernia. This ring is composed of normal esophageal epithelium, but may have a columnar mucosa on the gastric side. |
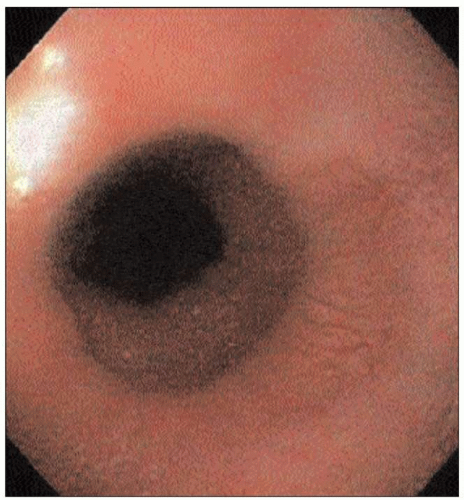 4.28 The ‘A’ ring is most often detected on barium swallow, and is rarely symptomatic. The location is approximately 2 cm proximal to the SCJ. |
Table 4.6 Etiology of esophageal strictures | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
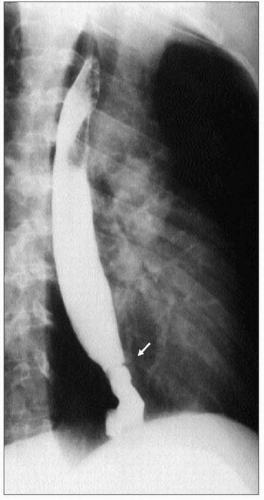 4.30 Barium esophagram of a peptic stricture (arrow). Peptic strictures usually occur in the distal esophagus and are associated with intermittent or progressive dysphagia. Strictures are treated with gentle dilation and PPI therapy. PPIs are superior to H2 blockers in preventing the recurrence of acidrelated strictures. |
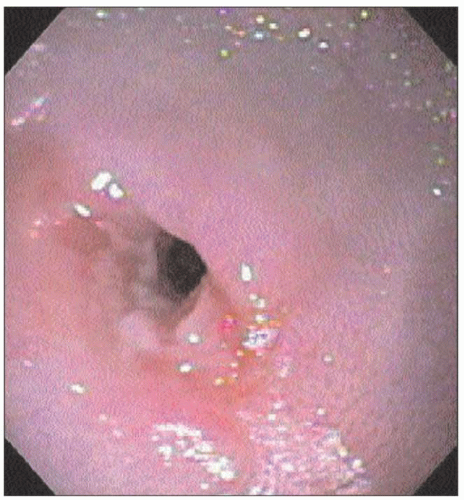 4.31 Endoscopic photograph of a tight radiationinduced stricture. Initially the standard upper GI endoscope was unable to pass through the narrow lumen. |
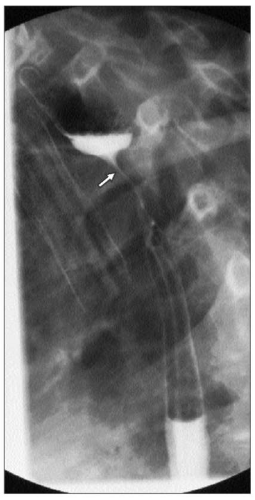 4.32 Barium esophagram of the stricture in 4.31 (arrow). Note the residual lumen is only several millimeters in width. Stricture length can also be determined easily based on this barium study. |
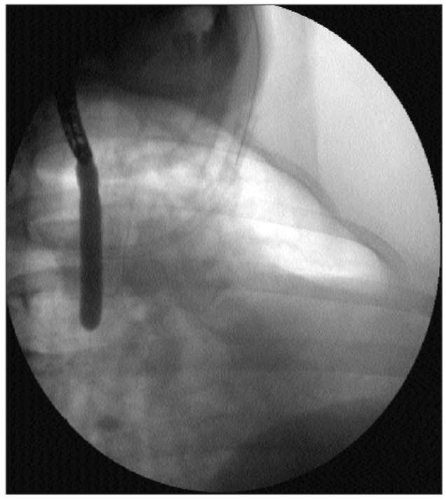 4.34 Fluoroscopic image of the dilation in 4.33. The balloon expands fully and there is no residual ‘waist’. |
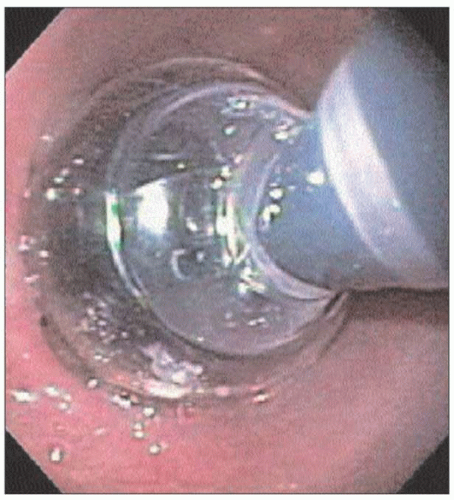
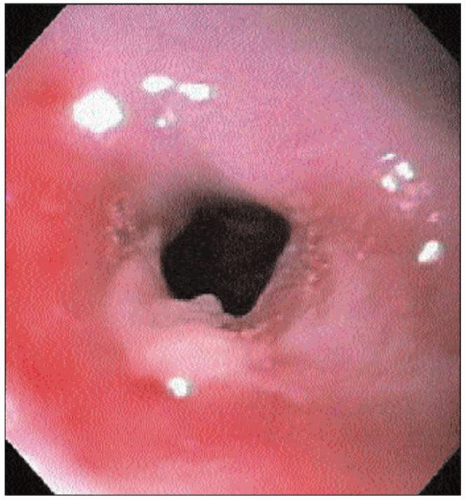 4.35 Endoscopic image post-dilation. The lumen is now larger, allowing for passage of the upper endoscope. |
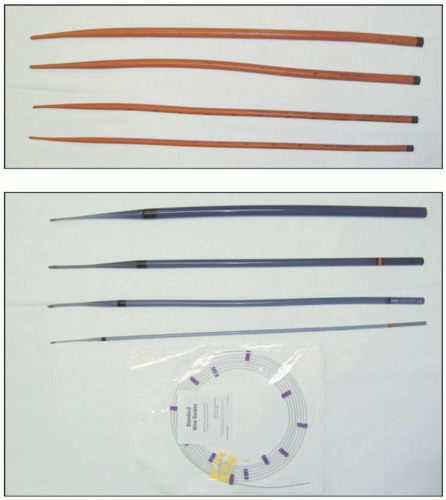 4.36, 4.37 There are several different types of dilators available (in addition to the ‘through-the-scope’ balloons as in 4.33). 4.36 Mercury-filled Maloney dilators (top). 4.37 Wire-guided rigid Savary-Gilliard dilators. Choice of dilator often depends on the anatomy of the stricture and operator expertise. In general, Maloney bougies are used in uncomplicated, short, straight strictures. The wireguided Savary-Gilliard and TTS balloons are best suited for long, tight, or tortuous strictures. |
Table 4.7 Epidemiology of esophageal cancer | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
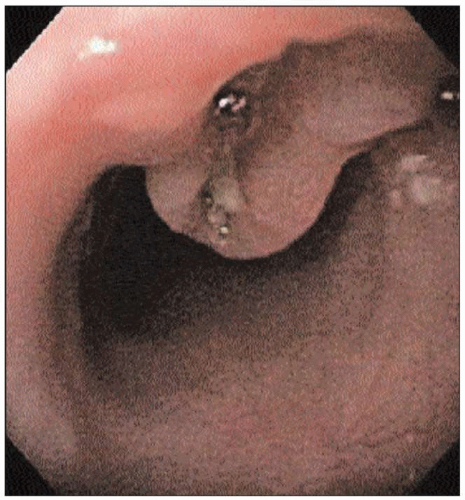 4.39 Endosocopic photo of an ulcerated midesophageal mass. Biopsy confirmed the presence of esophageal SCC. |
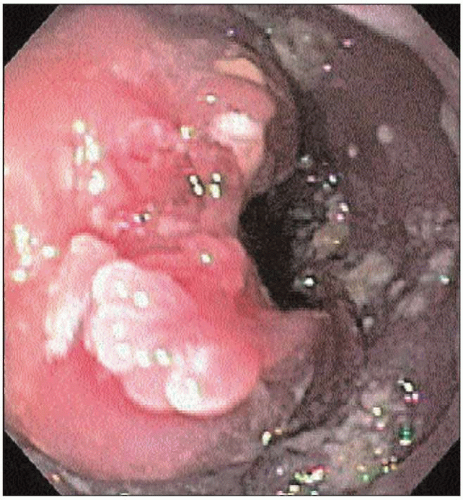 4.40 Endoscopic photo of a large mass present at the GEJ. Biopsy confirmed adenocarcinoma. |
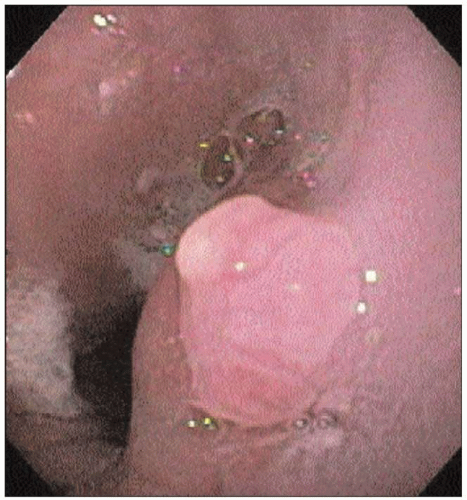 4.41 Endoscopic photo of a nodule arising at the proximal end of Barrett’s esophagus. Biopsy documented adenocarcinoma within the nodule. |
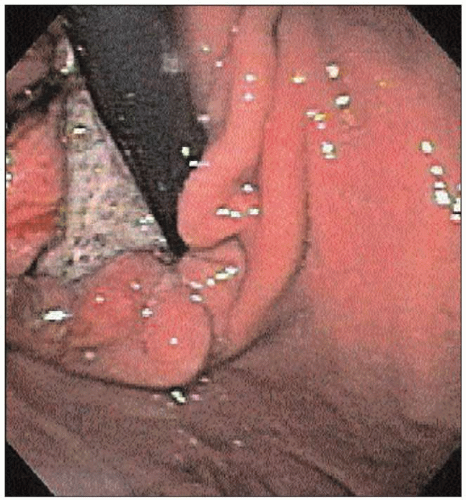 4.42 Retroflexed view of the GEJ. An ulcerated adenocarcinoma is seen. Tumors located at the GEJ can mimic the signs and symptoms of achalasia and is known as ‘pseudoachalasia’.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|