Authors
Publication year
Surgical approach
N
Leak rate(%)
Cervical anastomosis
Heitmiller et al. [17]
1999
a
262
0.8
Swanson et al. [42]
2001
Three field
342
8
Walther et al. [12]
2003
Three field
41
2.4
Luketich et al. [43]
2003
MIE
222
11.7
Orringer et al. [44]
2007
Transhiatal
944
9
Klink et al. [45]
2012
Transhiatal
36
31
Kassis et al. [5]
2013
Transhiatal
1050
11.6
Three field
519
14.3
MIE
168
10.1
Price et al. [46]
2013
a
163
21
Intrathoracicanastomosis
Visbal et al. [47]
2001
Ivor-Lewis
220
0.9
Walther et al. [12]
2003
Ivor-Lewis
42
0
Crestanello et al. [4]
2005
Ivor-Lewis
761
6.3
Ott et al. [48]
2009
Ivor-Lewis
240
8.3
Klink et al. [45]
2012
Ivor-Lewis
36
11
Kassis et al. [5]
2013
Ivor-Lewis
1174
9.3
Thoracoabdominal
105
5.7
MIE
280
10.7
Price et al. [46]
2013
b
269
5.9
Risk Factors for Anastomotic Leak
Risk factors for leaks are best considered when divided into technical and patient specific causes. Technical risk factors are perhaps the most easily modified and harken back to the basic tenets of any surgical anastomosis—minimizing tension while maintaining perfusion. First and foremost is careful preparation of the neoesophageal conduit and avoidance of conduit ischemia. Prevalence of conduit ischemia may be as high as 10 % [6]. Meticulous surgical technique in preservation of vascular supply of the conduit is vital to prevent conduit ischemia, and a major risk factor for leak is reflected in the surgical maxim “Pink in the belly, pink in the neck or chest.” Therefore, maintaining adequate arterial blood supply through preservation, in the case of gastric conduits, of the right gastroepiploic, and if possible the right gastric artery, is of paramount importance. To accomplish this, the greater omentum is separated from the stomach beginning high on the greater curve where the right gastroepiploic terminates. The omentum should then be divided proximally to the left crus, dividing the short gastric vessels. Distally, the omentum should be separated carefully while palpating the gastroepiploic artery to ensure it is not inadvertently injured.
Of equal importance, though often less emphasized, is preservation of venous drainage through careful dissection and preservation of the pars flaccida. The gastrohepatic ligament should be divided at its filmy attachments up toward the hiatus. The left gastric vein is identified and divided close to its origin, as is the left gastric artery. All adjacent lymph nodes and venous drainage along the lesser curve should be swept toward the stomach. Once ready to divide the stomach, the lesser curve should be divided at the level of the second vascular arcade, thereby preserving some of the venous drainage. The stomach should continue to be divided toward the gastric fundus while progressively stretching the stomach cephalad and straightening the gastric tube. Upon completion of the conduit, the stomach should be carefully inspected while still in the abdomen to ensure that it remains pink and perfused and to confirm a palpable pulse in the right gastroepiploic artery.
Key points in regard to minimizing tension on the anastomosis include adequate mobilization and careful tubularization of the stomach in order to create a conduit of sufficient length. Kocherizing the duodenum and division of any adhesions to the pancreas in the lesser sac will allow for a complete mobilization of the stomach. Additionally, division of the left gastric and short gastric arteries will further increase intraabdominal mobilization and length. Finally, tubularizing the stomach along the greater curvature will help create a straight conduit of sufficient length to reach the anastomosis while allowing for adequate drainage of the conduit. A conduit which is too wide will not have enough length and will not empty well predisposing to a leak. However, a narrow conduit (3 cm in width) is predisposed to increased ischemia at the gastric tip, likely due to the removal of collateral blood supply. Previous studies have shown the ideal conduit width to be 4–5 cm both in open and minimally invasive esophagectomy [7–9].
In the rare case in which stomach is unavailable, most commonly due to prior gastric surgery, colon or jejunum can be used as possible conduits. While both of these methods provide possible alternatives to stomach, they carry the disadvantages of two additional anastomoses and the risk of an intraabdominal leak. Use of colon interposition, while uncommon, has been studied in several retrospective series with equivalent, if not slightly lower, leak rates when compared with the stomach [6,10]. Both right and left colon with inclusion of the transverse colon can be used, though our preference is the left as it provides a better size match to the esophagus. Use of the colon often necessitates a preoperative colonoscopy to rule out colonic pathology and a CT angiogram to evaluate the colonic vessels, including the patency of the marginal artery. The blood supply to the left colon conduit will depend on the ascending branches of the left and middle colic arteries with a patent intervening marginal artery. Pulsation in all colonic arteries should be confirmed at the time of laparotomy. Mobilization of the peritoneal reflection from the splenic flexure down to the rectosigmoid junction is necessary in order to minimize tension. A 1- to 2-cm rim of mesocolon on the conduit should be maintained in order to preserve collateral blood supply.
Jejunum, on the other hand, lacks the risk of diverticular or malignant disease progression. Short segment jejunal interposition can be completed with relative ease when only a segment of distal esophagus needs reconstruction. When longer reconstruction is required, the use of a super-charged jejunum is necessary for added length though it requires the greater complexity of two microvascular anastomoses in addition to three enteric anastomoses. Recent data from the MD Anderson Cancer Center reported a leak rate of 32 % when utilizing this method [11].
Technical approaches to the esophageal-conduit anastomosis beyond the choice of conduit have been extensively studied as a possible risk factor of leak. Both the location and method of anastomosis have been looked at with prospective and retrospective studies. As previously mentioned, possible anastomotic locations are intrathoracic, as with Ivor-Lewis and thoracoabdominal esophagectomy, and cervical, as with transhiatal and McKeown three-hole esophagectomy techniques. A selection of studies reporting anastomotic leaks is shown in Table 3.1. Four randomized controlled trials have been conducted examining cervical vs. thoracic location [12–15]. A meta-analyses conducted by Markar et al. examining these studies ultimately concluded that leaks were significantly more common in the cervical group (13.64 %) than in the thoracic group (2.96 %) [16]. There continues to be a considerable variability in reported leak rates, however, with two studies reporting cervical leak rates less than 3 % [12, 17]. Given the additional length of conduit necessary to reach the neck, it is reasonable to assume that this increased leak rate is a result of increased tension and perhaps compromised blood flow at the conduit tip and possible decreased venous outflow due to conduit compression by the thoracic outlet. Nevertheless, it should be remembered that intrathoracic anastomotic leaks can be associated with considerable mortality and pulmonary complications, as opposed to a cervical anastomotic leak, which typically will present as a local wound infection requiring drainage only.
There has been considerable debate and study over the use of hand-sewn vs. partially stapled vs. circular-stapled anastomosis and their associated leak and stricture rates. Several prospective, randomized studies have been completed examining this with mixed results. Two separate meta-analyses analyzing 12 randomized, controlled studies have concluded no difference in leak rates between these methods (though stricturing does appear to be more common with the use of circular stapling) [16, 18]. Our practice, regardless of intrathoracic or cervical location, is to use a modified Collard technique, creating a partially stapled anastomosis where the posterior wall is created with a linear cutting stapler, and the anterior hood is closed using a single- or two-layer hand-sewn technique.
The other major technical factor often cited as a risk factor for anastomotic leaks has been the use of neoadjuvant chemoradiation therapy. It is logical to think anastomotic leak may be more common in this group as a result of radiation changes to the neoesophageal conduit, remaining native esophagus, and operative field. Conversely, the landmark CROSS trial, a randomized trial comparing patients undergoing esophagectomy with or without neoadjuvant chemoradiotherapy, found no significant difference in rates of anastomotic leakage [19]. A meta-analysis published in 2014 including 23 studies found no difference in rates of postoperative morbidity, including leakage rates [20]. Finally, several studies have examined the volume–outcome relationship for esophagectomy and have shown reduced postoperative mortality at high-volume centers [21]. However, very little work has been done examining the relationship between volume and postoperative complications, including anastomotic leaks, and will need further study.
In addition to these technical considerations, several patient-specific characteristics have been identified as risk factors for both anastomotic leak as well as overall morbidity after esophagectomy and are shown in Table 3.2. A multi-institutional Veterans Administration study identified the most important risk factors for morbidity after esophagectomy to be COPD, diminished functional health, advanced age, albumin less than 3.5 g/dL, alkaline phosphatase greater than 125 U/L, creatinine greater than 1.2 mg/dL, and prothrombin time of greater than 12 s [22]. Perhaps the most important risk factors for breakdown of the anastomoses are those that have a direct effect on tissue healing, namely diabetes, malnutrition, and steroid use. The use of epidural anesthesia has been found to be associated with decreased leak rates in one retrospective study [23], and several other studies have suggested diminished blood flow in the anastomotic end of a gastric tube after the administration of thoracic epidural bupivacaine [24, 25]. These seemingly dichotomous findings will need to be further examined in future studies before any definitive conclusion can be made.
Table 3.2
Risk factors for anastomotic leak
Technical factors | Patient-specific characteristics |
|---|---|
1. Cervical anastomosis | 1. Age |
2. Tension | 2. Diabetes mellitus |
3. Excessive intraoperative blood loss | 3. Steroid use |
4. Prolonged operation | 4. Congestive heart failure |
5. Compromised blood supply/venous drainage | 5. Hypertension |
6. Renal insufficiency | |
7. Poor nutritional status |
Presentation and Identification of a Leak
Clinical presentation of anastomotic leaks can be quite variable and can range from asymptomatic to severe sepsis. The severity of presentation is largely secondary to the size and location of the leak. Urschel et al. proposed a frequently cited four-category classification in 1995: clinically silent leak, early fulminant leak, clinically apparent thoracic leak, and clinically apparent cervical leak [26]. This classification system provides a convenient framework to discuss the presentation and identification of post-esophagectomy anastomotic leaks.
Clinically silent leaks are those found on imaging studies alone. Routine gastrograffin/barium esophagram is often pursued one week after esophagectomy by many surgeons, ourselves included. These imaging studies will occasionally show extraluminal extravasation of contrast material into a contained collection (Fig. 3.1). Other methods of detection include careful physical examination, chest radiograph showing new right pleural effusions in transthoracic esophagectomy, and CT scan. These leaks are typically the result of a small defect in the anastomosis itself. As a result, patients with clinically silent leaks will often remain asymptomatic, or only have subtle clinical findings missed at first glance, such as low-grade fevers and mild tachycardia. Left untreated, these leaks will continue to progress and can lead to significant morbidity. Esophageal surgeons must therefore maintain a high index of suspicion in order to not miss these often imperceptible findings.
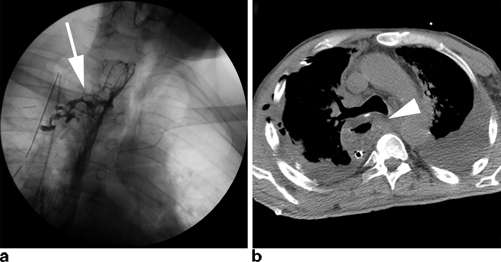

Fig. 3.1
Commonly used imaging studies to evaluate for an anastomotic leak include contrast esophagram (a) and CT scans (b). Esophagrams may show contrast extravasation freely into the chest or into a contained leak ( arrow). CT scans may have a number of findings including worsening pleural effusions, esophageal thickening ( arrowhead), or possible contrast extravasation
Early fulminant leaks are the most life-threatening manifestations of anastomotic leaks. They are typically the result of complete or near-complete necrosis of the neoesophagus, usually due to compromised arterial blood supply or venous drainage. Careful preparation of the gastric conduit is of paramount importance in order to avoid this dread complication. These patients will typically present in profound vasodilatory shock within 48–72 h of esophagectomy. Prompt recognition, resuscitation, and operative intervention are required in order to avoid significant morbidity and/or death.
Clinically apparent thoracic leaks in patients undergoing Ivor-Lewis esophagectomy can be a source of significant morbidity. Presentation can vary considerably. Possible signs include changes in character or quantity of chest tube drainage, new pleural effusions or pneumonias, worsening chest pain, fever, tachycardia, new onset atrial fibrillation, and worsening leukocytosis. Any change in clinical status must therefore be assumed to be the result of an anastomotic leak until proven otherwise. Late leaks may present as a fistula to the trachea or right main stem bronchus with recurrent pneumonias and aspiration of gastric contents (Fig. 3.2). Lastly, clinically apparent cervical leak after transhiatial or three-hole esophagectomy will typically present with low grade fevers, new-onset atrial fibrillation, neck erythema and cellulitis, severe halitosis, and possibly purulent drainage. Prompt recognition on physical exam is again of utmost importance.
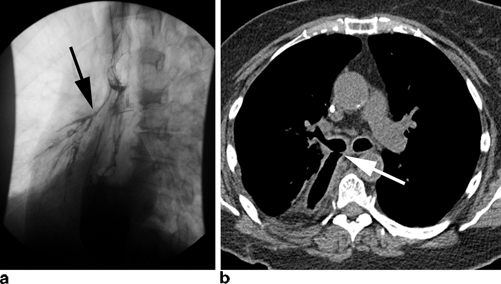

Fig. 3.2
Chronic leaks can fistulize to adjacent organs including the tracheobronchial tree as seen here on esophagram ( black arrow) (a) and CT scan ( white arrow) (b)
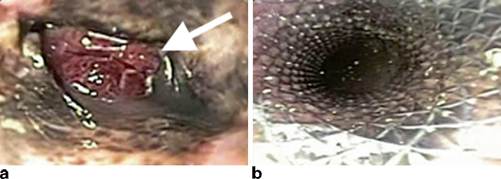
Regardless of classification, identification of an anastomotic leak requires a high index of suspicion in order to recognize the subtle early clinical findings mentioned previously. Several imaging studies can help confirm the diagnosis of a leak. The most commonly used study is a contrast esophagram with gastrograffin followed by barium in order to improve sensitivity (Fig. 3.1a ). Unfortunately, the sensitivity of contrast swallow studies for routine identification of a leak has been reported as low as 45− 80 %, and as many as 40 % of leaks may be missed [27, 28]. For this reason, many centers no longer obtain routine esophagrams. Our practice continues to be obtaining an esophagram on the seventh postoperative day to evaluate gastric emptying as well as to evaluate for anastomotic leakage. CT scans may reveal any number of findings consistent with a leak such as a new pleural effusion, esophageal thickening, or possible contrast extravasation (Fig. 3.1b ). As a result, however, the specificity of these findings for a leak is significantly lower than esophagram. Nevertheless, one study has shown that the addition of a CT scan to a contrast swallow can increase sensitivity and negative predictive value for the identification of a leak to 100 % [28]. Other useful imaging tests include upper endoscopy, which allows for direct visualization of the degree of mucosal involvement and quantification of amount of healthy conduit remaining (Fig. 3.3). Additionally, it has the added advantage of allowing for possible therapeutic interventions such as stenting and dilation as will be discussed later in this chapter.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







