Chapter 14 Endoscopic ultrasound of the biliary tract and pancreas
Imaging and Diagnosis
The diagnosis of benign and malignant diseases of the pancreas and biliary tree has traditionally relied on a detailed history and complete physical examination, with correlation of the results of clinical chemistries. Imaging of the hepatic and pancreatic parenchyma and ductal anatomy has, however, evolved as critical for accurate diagnosis and for guiding therapy. Routine radiography does not provide the soft-tissue resolution required, but ultrasonography (US; see Chapter 13), computed tomography (CT; see Chapter 16), and magnetic resonance imaging (MRI; see Chapter 17) have become important noninvasive modalities in the routine investigation of almost any symptom, physical sign, or laboratory abnormality potentially relating to pathologic conditions of the liver or pancreas.
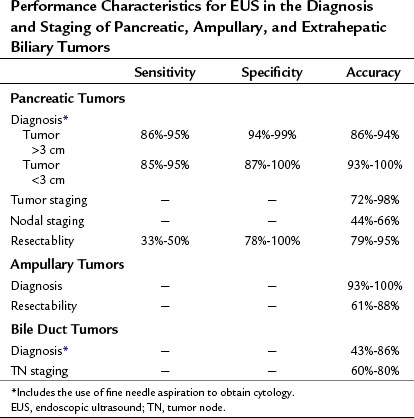
Invasive procedures for imaging the biliary and pancreatic ductal systems, primarily percutaneous cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP) remain important therapeutically, but with respect to diagnosis, these have been almost entirely replaced by noninvasive modalities. Endoscopic ultrasonography (EUS) has also become an important tool for diagnosis and treatment. Its high-resolution images complement the more general findings of cross-sectional imaging and result in a higher sensitivity for diagnosis of early-stage disease. The new-generation EUS instruments (Figs. 14.1 and 14.2) permit guided passage of needles and devices through the endoscope, allowing biopsies to be obtained and permitting therapeutic interventions. This chapter discusses the techniques of radial and linear endosonography in the diagnosis, staging, and treatment of benign and malignant disease of the pancreas and biliary tree.
Endoscopic Ultrasound Technique
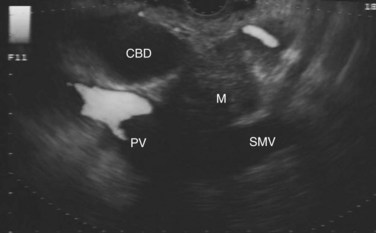
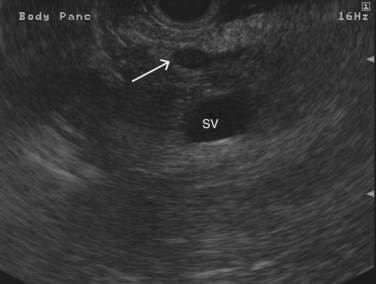
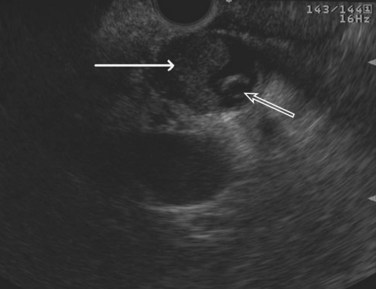
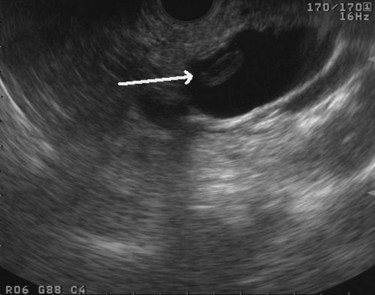
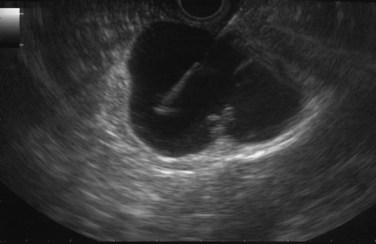
The normal pancreatic parenchyma has a homogeneous echogenic appearance (Fig. 14.3), and tumors usually appear hypoechoic, often with irregular borders, in sharp distinction from normal tissue (Fig. 14.4). Large tumors of the pancreas may be difficult to evaluate completely because of limited penetration of the tumor by US. Conversely, small tumors of the pancreas that are often missed by CT or MRI are readily imaged by EUS. For example, islet cell tumors, which are often encapsulated and small, are frequently detected on EUS and appear as well-demarcated hypoechoic lesions (Fig. 14.5). Other neuroendocrine tumors, such as gastrinomas, may be isoechoic within the pancreatic parenchyma and difficult to identify without careful, tedious, real-time imaging. Ampullary tumors are also often seen and staged on EUS because of their relation to the duodenal wall, CBD, and pancreatic duct (Fig. 14.6). Extrahepatic bile duct tumors can also be detected and described in detail with EUS imaging (Fig. 14.7).
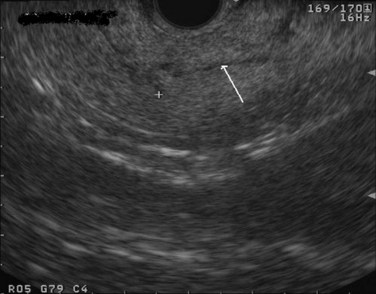
FIGURE 14.3 Normal endoscopic ultrasound image of the body of the pancreas with thin main pancreatic duct (arrow).
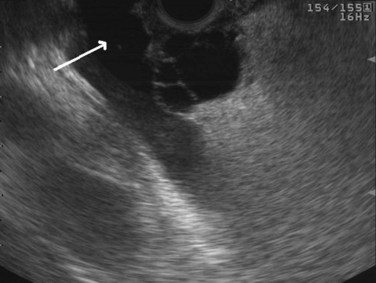
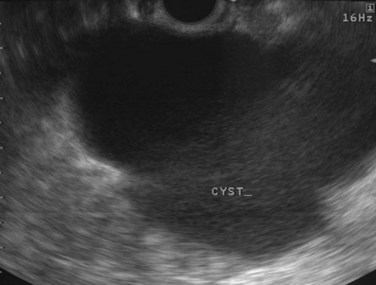
Cysts of the pancreas are generally anechoic and well demarcated and thus easily identified even when small. Some cysts may have internal echoes or solid areas, which raise concern for a mucinous lesion or associated tumor (Fig. 14.8). If the cysts are complex with septa (Fig. 14.9), they are best distinguished from vascular structures using the Doppler flow feature of the linear array echoendoscopes. Serous cystadenomas may also appear isoechoic with the pancreas and require careful imaging for proper identification (Fig. 14.10). Pseudocysts can vary in size and sonographic features but usually lack a discrete wall and septa, particularly if acute. However, internal echoes often are seen as a result of necrotic debris (Fig. 14.11).
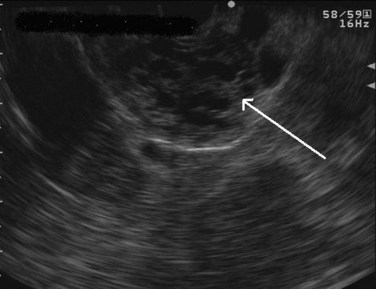
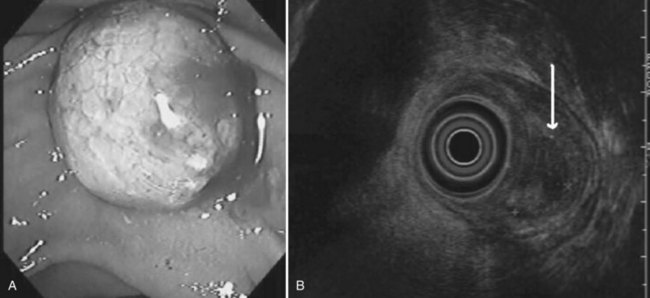
FIGURE 14.10 Typical microcystic appearance of a serous cystadenoma in the head of the pancreas (arrow).
Endoscopic Ultrasound-Guided Fine Needle Aspiration
Endoscopic Ultrasound Fine Needle Aspiration Technique
Within the duodenum or stomach, the US probe is positioned in close proximity to the target lesion, typically less than 3 cm away. The area is then interrogated with Doppler flow US to ensure the absence of significant vascular structures in the needle path. A 25-, 22-, or 19-gauge needle can then be directed into the target lesion. The tip and shaft of the needles used for FNA are manufactured to produce a bright, hyperechoic image. This allows the needle to be followed in real time to ensure precise positioning within the target lesion (Fig. 14.12). Ideally, a cytopathologist or cytotechnologist should be present at the time of the FNA to determine the cellular adequacy of the specimen. Alternatively, multiple punctures (up to seven) should be performed to ensure an adequate cytologic specimen (LeBlanc et al, 2004). Cyst fluid can also be aspirated and sent for cytology, tumor markers, and chemical analysis (Fig. 14.13).
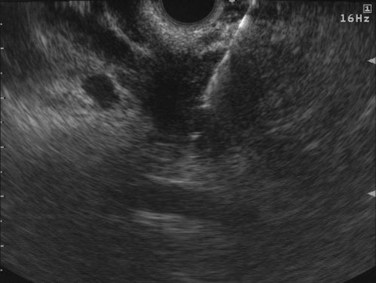
FIGURE 14.12 Bright appearance of fine needle aspiration of a solid mass in the head of the pancreas.
Diagnosis of Pancreatic Cancer
Endoscopic Ultrasound Fine Needle Aspiration of Solid Pancreatic Lesions
Solid masses of the pancreas may represent a primary pancreatic cancer, neuroendocrine tumor, metastatic lesion, or focal pancreatitis. These masses may be difficult to visualize and are often indistinguishable on noninvasive imaging. Percutaneous CT-guided biopsy has a pooled sensitivity of 87% and negative predictive value of only 58% (Hartwig et al, 2009), and it carries the risk of cutaneous or peritoneal tumor seeding. Therefore a percutaneous biopsy is best indicated in patients with unresectable disease to help establish a diagnosis and plan palliative care. Similarly, the sensitivity of cytology obtained from brushings during cholangiography is poor (approximately 40%) and cannot adequately exclude cancer (Wakatsuki et al, 2005).
EUS-FNA is highly accurate in diagnosing patients with suspected pancreatic cancer with a sensitivity of 77% to 95%, specificity of 94% to 99%, positive predictive value (PPV) of 98% to 100%, and a negative predictive value (NPV) of 86% to 92% (Wiersema et al, 1997; Yusuf et al, 2009; Turner et al, 2010; Table 14.1). EUS-FNA should be considered as the initial approach to lesions in or adjacent to the pancreas. In a prospective study of 102 patients with suspected pancreatic cancer who had a negative CT-guided biopsy, EUS-FNA had a sensitivity of 95%, specificity of 100%, PPV of 100%, and a NPV of 92% (Gress et al, 2001). With EUS, the issue of needle-tract tumor seeding is minimized, as the FNA is generally performed through a segment of duodenal or stomach wall that will be removed as part of a resection, should the patient be an appropriate surgical candidate. The utility of preoperative EUS-FNA was further demonstrated in a prospective study of 44 patients with resectable pancreatic mass lesions by cross-sectional imaging. EUS with or without FNA precluded surgery in 27% of patients by determining vascular invasion and nodal involvement or by diagnosing lymphoma of the pancreas (Chang et al, 1997).
Endoscopic Ultrasound Fine Needle Aspiration of Pancreatic Cystic Lesions
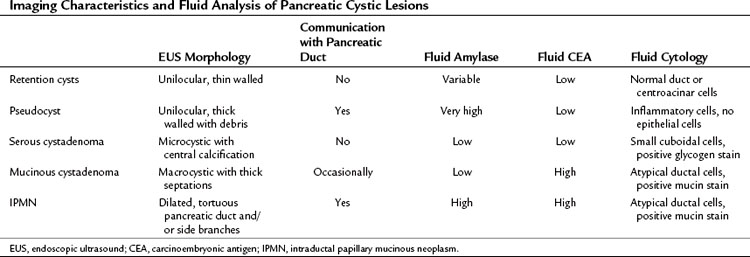
Cystic lesions of the pancreas remain a diagnostic and therapeutic challenge. The differential diagnosis includes retention/simple cysts, pseudocysts, cystic neoplasms, and cystic degeneration of solid masses (Table 14.2). EUS provides detailed information about the entire pancreas—location and number of cysts, size, ductal dilation or communication, signs of chronic pancreatitis, cyst wall thickness, mural nodules, papillary projections, and intracystic structures such as septations, debris, and mass component. Retention cysts are generally small, thin-walled, and unilocular; they carry no malignant potential and can be left untreated. Pseudocysts develop as a result of acute inflammation. On EUS, they are thick-walled as they become chronic and may have debris within the cyst cavity (see Fig. 14.11). They can be seen in or adjacent to the pancreas and often communicate with the pancreatic duct. FNA will yield thick fluid with inflammatory cells but no epithelial cells, and tumor marker levels (carcinoembryonic antigen [CEA], CA19-9) in the cyst fluid are low or undetectable; by contrast, fluid amylase is usually markedly elevated.
Regarding cystic neoplasms, it is important to distinguish mucinous and papillary tumors from serous lesions (see Chapter 57). Serous tumors have little or no malignant potential and do not require resection unless symptoms occur. Typically, serous cystadenomas are composed of a honeycomb of microcysts with no communication with the pancreatic duct (see Fig. 14.10). They can be large and exhibit central stellate calcification on imaging studies. Papillary lesions, solid and pseudopapillary tumors, carry the risk of malignant transformation and usually should be referred for resection. Mucinous lesions—mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN)—also harbor malignant potential that appears to correlate with size; however, universally accepted management criteria are lacking, and treatment recommendations often vary. Mucinous cysts are generally composed of one or more large cystic spaces with thickened walls or septa (see Fig. 14.9). In the case of IPMNs, distinguishing main-duct from side-branch types is important clinically, because the former are associated with a much higher risk of malignant change. Cross-sectional imaging may help in this regard by demonstrating a dilated pancreatic duct characteristic of main-duct IPMN. However, diagnostic uncertainty is not uncommon, and communication with the main pancreatic duct may be demonstrated on EUS.
Although these anatomic characteristics are helpful, EUS cyst morphology alone is insufficient to characterize the lesion (Ahmad et al, 2003; Brugge et al, 2004). Aspiration of cyst fluid via EUS-FNA can further aid in the diagnosis. Cyst fluid can be analyzed for tumor markers, amylase, and molecular markers, and it provides material for cytopathologic assessment. Because of the low cellularity of cyst fluid, the sensitivity and negative predictive value of cytology is low (Centeno et al, 1997; Sedlack et al, 2002). The presence of thick, viscous fluid and a positive mucin stain is suggestive of a mucinous lesion. The concentration of several tumor markers in pancreatic cyst fluid has been examined, and CEA has been found to be the most useful. A cyst-fluid CEA concentration of more than 192 ng/mL predicts a mucinous lesion with a sensitivity of 73%, specificity of 84%, and an accuracy of 79% (Brugge et al, 2004).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree