Fig. 3.1
Normal anatomy of the pancreas using a linear echoendoscope (a) head of the pancreas with the SMV/PV confluence. (b) Body of the pancreas with the main pancreatic duct (c) imaging of the celiac trunk and celiac artery take-off
Perhaps in no other disease state has the widespread use of the linear echoendoscope become more apparent than in pancreatic cancer. In addition to vascular staging, the immediate advantage of tissue confirmation is implicit. Initial primary staging, followed by FNA of either the pancreatic mass itself or of lymph nodes, to the ultimate ability to deem the patient as having unfortunate distant metastasis to organs such as the liver via tissue confirmation at the same setting make the linear echoendoscope the preferred instrument. Using a “station approach,” one can obtain excellent views of the uncinate pancreas (with the EUS scope tip near the ampulla), head of the pancreas (with the EUS scope tip in the duodenal bulb), body of the pancreas (with the EUS scope tip in distal stomach), and tail of the pancreas (with the EUS scope tip in the gastric fundus). The left lobe of the liver is best seen with the scope along the lesser curve of the stomach and the scope tip at the body/antrum junction. Metastatic lesions to the left lobe can be easily seen, especially those abutting the gastric wall and amenable to FNA (Fig. 3.2). The working channel (which varies in diameter from 2 to 3.8 mm) is designed so that as the FNA needle is advanced, it will be within the plane of scanning and can be visualized as it enters the target tissue.
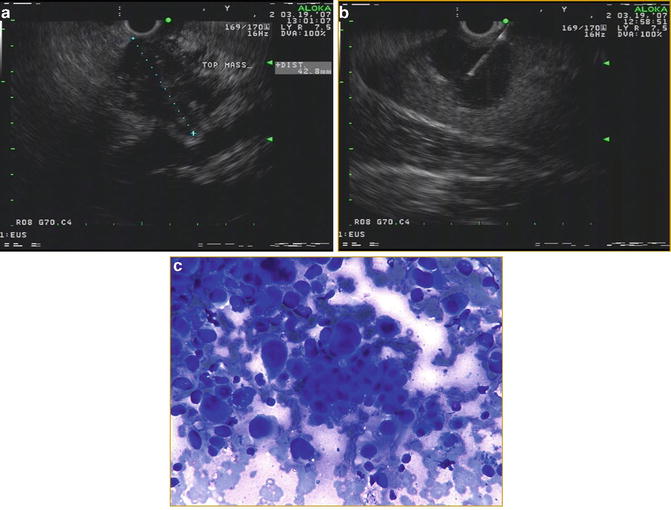

Fig. 3.2
(a) EUS image of an approximately 4.2 cm mass in the tail of the pancreas with irregular borders (b) FNA using a 25-gauge needle of a large metastatic lesion in the left lobe of the liver (c) cytology from the liver showing large, irregular cells consistent with metastatic adenocarcinoma
Radial Versus Linear Echoendoscope
Several studies emerged in the late 1990s that specifically compared radial scanning with the linear array for primary staging of pancreatic cancer as well as the utility of the linear array scope in both benign and malignant pancreatic lesions. The first study performed by Gress et al. utilized a cohort of 79 patients referred with pancreatic cancer [6]. As only 33 patients ultimately had surgical excision, the evaluable groups consisted of 17 patients randomized to linear array and 16 to radial scanning EUS. EUS staging accuracy for linear array was 94 % (16 of 17) for T and 71 % (12 of 17) for N staging. The staging accuracy for radial scanning was 88 % (14 of 16) for T and 75 % (12 of 16) for N staging. Surprisingly, radial scanning was more accurate for predicting vascular invasion 100 % (16 of 16) than the linear array 94 % (16 of 17). More recent literature militates against EUS superiority in assessing for vascular invasion. Seicean et al. explored the staging accuracy of radial EUS in pancreatic cancer and how well EUS can predict tumor resectability in 30 patients with pancreatic masses staged by both a radial EUS and surgery [7]. Resectability was based on EUS involvement of either the celiac trunk or superior mesenteric artery. Specific EUS criteria for vascular invasion were defined as direct visualization of loss of hyperechoic vascular wall, tumor ingrowth with complete vascular obstruction or the presence of peripancreatic venous collaterals. The accuracy of EUS T staging was 86.6 %, N staging was 93.3 %, while that of vascular invasion was 80 %. These authors advised using adjunct imaging modalities to a radial EUS for assessing arterial invasion. Conventional radial and linear echoendoscopes offer 2D imaging. However, 3D imaging would be ideal when assessing for vascular invasion. Fritscher-Ravens et al. describe their experience using a linear echoendoscope to identify a pancreatic tumor followed by 3D image acquisition using a magnetic tracked 3D sensor [8]. The EUS results from 22 patients with solid pancreatic lesions (17 adenocarcinomas, 5 chronic pancreatitis) were compared to surgical histology. In 30 % of cases, the 3D reconstruction suggested vascular compression rather than vascular invasion. One patient was incorrectly classified as having vascular invasion with the 3D reconstruction. Although 3D reconstruction is largely investigational, this study is tantalizing for the prospect of achieving greater clarity of vascular involvement by EUS preoperatively.
EUS Versus Other Imaging Modalities
The initial excitement of the superior imaging qualities provided by EUS for pancreatic cancer has now been tempered by the reality that newer generation computed tomography (CT) scanners with 3D reconstruction allow for superb imaging and, thus, staging. Nonetheless, reviewing the literature offers insights as to the relative merits for each technology. A landmark study by DeWitt and colleagues compared EUS and multidetector CT for the detection, staging, and resectability of known or suspected locoregional pancreatic cancer using a prospective, observational cohort [9]. EUS followed by multidetector CT was performed in 104 patients; patients with known or suspected pancreatic cancer felt to be resectable by 1 or both tests were considered for surgery. Of the 80 patients with pancreatic cancer, 27 (34 %) were managed nonoperatively and 53 (66 %) underwent surgery (n = 25, resectable; n = 28, unresectable). The sensitivity of EUS for detecting a pancreatic mass was 98 % [95 % CI, 91–100 %]; the sensitivity for CT was 86 % [CI, 77–93 %]; p = 0.012. EUS was superior to CT for tumor staging accuracy (67 % vs. 41 %; p < 0.001) but equivalent for nodal staging accuracy (94 % vs. 47 %; p > 0.2). In the 25 patients recommended for surgery, EUS correctly identified 88 % of patients whereas CT correctly identified 92 % of patients. In the 28 unresectable pancreatic tumors in the patients recommended for surgery, EUS and CT correctly identified 68 % and 64 %, respectively. The investigators teased out characteristics of masses undetected by EUS (n = 2) and CT (n = 10) in patients undergoing surgery. Nine of these missed lesions by CT were ≤25 mm. The authors’ conclusions echoed most diagnosticians in the field that a preoperative EUS should be performed when a CT fails to detect a mass in patients with suspected pancreatic cancer (Fig. 3.3).
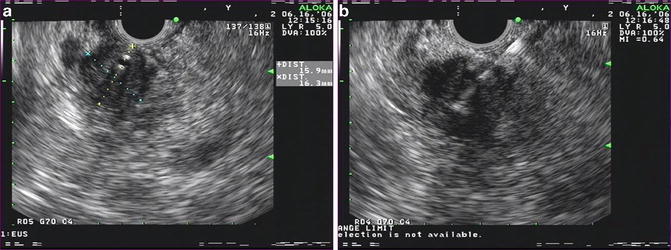

Fig. 3.3
(a) Linear EUS image of an approximately 1.6 × 1.6 cm mass in the pancreatic head with a biliary stent. This lesion was not detected by CT (b) FNA of pancreatic head mass, positive for adenocarcinoma
As mentioned above, EUS allows for detailed imaging to assess for vascular involvement prior to planned surgery. However, recent studies question the ability of expert endosonographers to accurately assess for vascular involvement [10, 11]. Rösch and colleagues showed videotapes of 75 patients with cancer involving the pancreatic head and asked to assess for involvement of either the portal vein confluence, superior mesenteric vein, or the celiac axis. The investigators withheld clinical information from the EUS experts to exclude potential bias. The sensitivity and specificity of EUS in the diagnosis of venous invasion were 43 % and 91 %, respectively, when using parameters such as visualization of tumor in the lumen, complete obstruction, or collateral vessels. The portal vein confluence was the only vascular system that could be properly visualized. A more recent study from Aslanian and colleagues compared EUS with surgical findings of vascular adherence and the pathology finding of vascular invasion among patients undergoing resection of pancreatic masses [12]. Vascular adherence was defined as tumor adherence requiring vascular resection and vascular invasion was defined as histologic invasion of vessel wall by tumor. They used both the radial and linear echoendoscopes. Thirty of 68 patients were eventually resectable. Sensitivity, specificity, PPV, and NPV of EUS were 63 %, 64 %, 43 %, and 80 % for vascular adherence and 50 %, 58 %, 28 %, and 82 % for vascular invasion, respectively. Similar to Rösch’s study, Aslanian and colleagues found that the NPV rose to 90 % for vascular adherence if only the portal confluence was considered.
An in-depth evaluation of the ability of EUS and CT to predict an R0 resection is absolutely germane as we constantly strive to offer our patients the greatest opportunity for cure and long-term survival when dealing with pancreatic cancer. Data derived from both CT and EUS are often necessary to determine the best treatment options, often with a multidisciplinary approach. The complementary roles for both EUS and CT are best highlighted by a recent study specifically investigating the ability of EUS and CT to predict a margin-negative R0 resection in patients undergoing a pancreaticoduodenectomy [13]. A linear echoendoscope was used in 76 patients. The endosonographers were blinded to prior imaging results and clinical outcome and reviewed either a video or the procedure note with pictures and noted the involvement of the superior mesenteric vein and artery, celiac axis, hepatic artery, portal vein, lymph nodes, and liver. Their definitions were similar to those used by prior investigators and included vessel abutment (loss of the hyperechoic interface between the tumor and vessel), vessel invasion (visualization of tumor within the lumen), vessel encasement, and vessel occlusion (Fig. 3.4). Using a subgroup of 27 evaluable patients who did not have a biliary stent, and using the above criteria, when EUS detected either venous invasion or abutment, there was an association with incomplete resection giving a sensitivity, specificity, PPV, NPV, and accuracy of 79 %, 69 %, 73 %, 75 %, and 74 %, respectively. In line with other studies, Bao and colleagues noted that EUS findings of arterial involvement were inaccurate, with three of six patients achieving an R0 margin despite the EUS report.
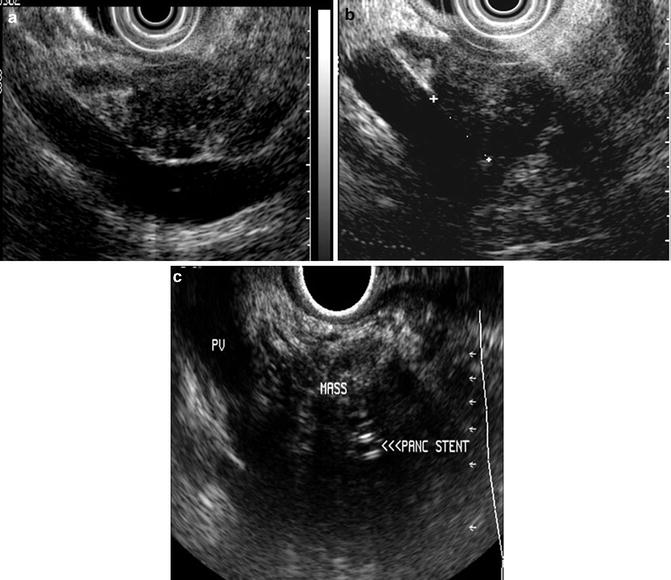

Fig. 3.4
EUS interpretation of vascular invasion (a) resectable pancreatic head mass with abutment (b) resectable pancreatic head mass with a short segment of adherence to the portal vein (c) unresectable pancreatic head mass due to portal vein invasion. A pancreatic stent is noted within the mass. (Images courtesy of Dr. Shyam Varadarajulu)
Clinically, it is not uncommon to attempt EUS staging after the placement of a transpapillary biliary stent by endoscopic retrograde cholangiopancreatography (ERCP) for malignant biliary obstruction. However, biliary stents can cause acoustic reverberation and shadowing, artifacts that can distort the outer margins of a mass, hampering the excellent views critical for EUS staging. This technical impediment was supported by a few studies that cautioned against the high accuracy rates for EUS staging [14, 15]. In fact, Kim et al. found that patients with biliary stents had lower accuracy of EUS-FNA for malignancy (77 %) than those without a biliary stent (89 %). These reports subsequently led to studies to specifically address the potential negative impact of a biliary stent (both plastic and metal) on staging accuracy and diagnostic yield for FNA [16–19]. These studies are limited due to retrospective study designs. However, the findings from these studies uniformly and consistently conclude that neither a metal nor a plastic biliary stent hampers accurate EUS staging or the high yield of EUS-FNA.
So, how does EUS compare to CT and other imaging modalities for staging of pancreatic cancer after factoring in the relative strengths and weaknesses of both modalities? Advances in cross-sectional imaging allow detailed views of the pancreas and the surrounding vasculature to warrant its use as a first-line modality for detection and staging when pancreatic cancer is suspected [20, 21]. Meta-analyses comparing these two technologies highlight the methodological limitations, heterogeneity in study design, quality, and results [22–24]. Interpretation of the results is further marred by the lack of technical uniformity in the CT scans and echoendoscopes used in the differing studies. These studies do, however, favor a slightly increased sensitivity for EUS in detecting pancreatic lesions less than 3.0 cm. If pancreatic cancer is detected with evidence of distant metastases, then there is no role for EUS outside of a research protocol or need for tissue acquisition prior to planned therapy. If however, the CT does not show a mass and the clinical suspicion for pancreatic cancer is high, then an EUS should be performed at which time an FNA can also be performed [25, 26].
Finally, and of great consolation for our patients and healthcare providers is the finding of a normal EUS when searching for a pancreatic cancer. EUS is associated with the best negative predictive value in patients with suspected pancreatic cancer [27–30]. Klapman and colleagues retrospectively reviewed nearly 700 patients with suspected pancreatic cancer who underwent EUS over a 4-year period. The NPV of EUS in excluding pancreatic cancer was 100 % over a mean follow-up of 25 months (range 8–48 months). In addition, 88 % of patients required no further work-up following the EUS.
Adjunct EUS Methods to Improve Detection
Contrast Enhancement
Contrast enhanced (CE) images are routinely used to better visualize mass lesions as well as the vasculature during cross-sectional imaging. Typically, malignant pancreatic masses present as hypoechoic lesions on EUS. However, benign pancreatic masses (either inflammatory or autoimmune) can often be difficult to distinguish on imaging, thus, necessitating an FNA. Intravenous US agents consist of microbubbles filled with heavy gases that allow for better visualization of the blood supply [31]. Contrast enhanced EUS (CE-EUS) allows for noninvasive visualization of the blood flow in small vessels as well as the microvasculature surrounding the target lesion of interest using harmonic imaging (CEH-EUS) [32]. Many of the commercially available agents used for enhancement in conventional ultrasonography cannot be used with EUS—the smaller transducer size and acoustic power of EUS limits use of certain agents. In a pilot study with 35 patients, Napolean and colleagues used an intravenous injection of a second-generation ultrasound contrast agent SonoVue®, (Bracco, Milan, Italy) along with a new Olympus prototype echoendoscope capable of detecting extended harmonics (XGF-UCT 180, Olympus America, Melville, NY) [33]. Sixteen of the 18 adenocarcinomas gave a hypointense signal on CEH. The sensitivity, specificity, NPV, PPV, and accuracy of hypointensity for diagnosing pancreatic adenocarcinoma were 89, 88, 88, and 89 %, compared with 72, 100, 77, 100, and 86 % for EUS-FNA. Several other studies have shown similar results—a hypoechoic mass on CEH-EUS was highly sensitive for pancreatic adenocarcinoma [34, 35]. In fact, Fusaroli et al. found that hyperenhancement specifically excluded adenocarcinoma (98 %).
Sonoelastography
The advent of newer needle designs when combined with greater experience by endosonographers and cytopathologist have led to phenomenal yield by EUS- FNA for diagnosing pancreatic cancer. However, false-negative results between 20 and 40 % have been reported in technically challenging cases or in the setting of inflammatory masses such as chronic pancreatitis [36–38]. The ability to obtain “virtual biopsies” without FNA in order to distinguish benign from malignant solid pancreatic masses would fill the void of false-negative samples [39]. Elastography represents a novel addition to EUS in an ongoing quest to improve imaging and help differentiate benign from malignant pancreatic masses technology that has evolved over the past 5 years. This technology can be applied with real-time ultrasound and relies on the firmness or elasticity of a given tissue relative to the adjacent normal tissue by measuring the strain or displacement generated in response to compression or vibration [40]. Conventional linear-array echoendoscopes (Pentax Medical, Montvale, NJ), an ultrasound platform (Hitachi 7500 or 8500, Hitachi) with an integrated elastography module can be used such that inflammatory masses or tumors will appear hard [41]. The elastography image can be superimposed over the conventional B-mode imaging such that hard tissue is depicted as blue, soft tissue in red, and tissue of intermediate elasticity as green-yellow (Fig. 3.5). This is termed qualitative elastography. Second-generation elastography or quantitative elastography attempts to remove the subjectivity by utilizing the ratio of the elasticity of a given mass to that of a selected region within adjacent soft tissue, termed the strain ratio (SR) [40]. Iglesias-Garcia et al. reported a 100 % sensitivity for both qualitative and quantitative elastography in 86 consecutive patients who underwent an EUS for the evaluation of solid pancreatic masses for distinguishing malignancy [42]. They reported an overall accuracy of 90.7 % using qualitative elastography and 97.7 % using strain ratio. Subsequent studies have not been able to replicate the same impressive performance characteristics suggesting that at present, elastography cannot replace pancreatic tissue sampling [40]. Meta-analyses of EUS elastography underscore the somewhat contradictory findings using this technology [43, 44].
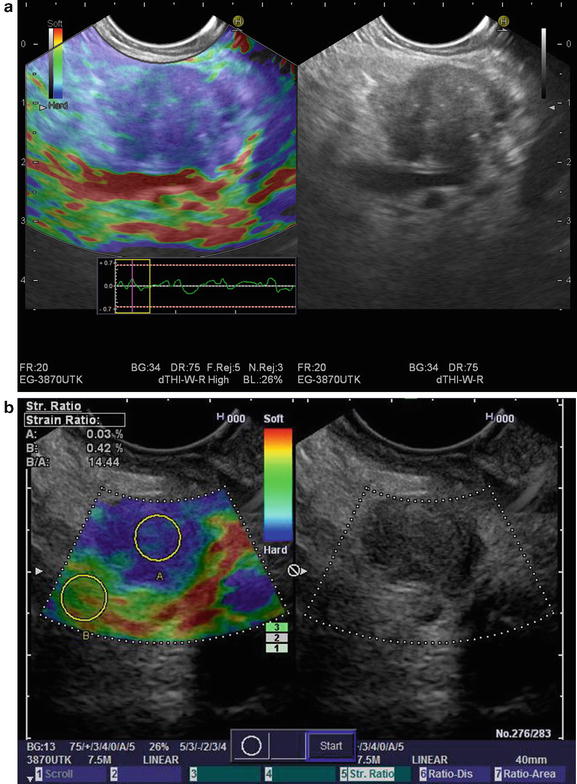

Fig. 3.5
(a) Sonoelastography images of a malignant pancreatic mass (b) sonoelastography images of malignant pancreatic mass with strain ratios (Images courtesy of Pentax Medical)
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) allows real-time imaging of the GI tract at approximately 1000-fold magnification such that the endoscopic images resemble light microscopy with resolution of approximately 1 μm [45]. Tissue illumination via a laser and subsequent detection of the fluorescence of light reflected through the pinhole increases the spatial resolution of CLE allowing for in vivo real-time histopathology of the mucosal layer [46]. The two clinically available systems are either endoscopic (eCLE) manufactured by Pentax (Pentax America, Montvale, NJ) or through-the-scope probe (pCLE) (Cellvizio, Mauna Kea Technologies, Paris, France). eCLE has been used to detect dysplasia and neoplasia in Barrett’s esophagus, classification of polyps, and assessment of resection margins after polypectomy, and evaluation of inflammatory disease [45, 47–51]. pCLE can be used for the above conditions but has steadily gained more acceptance for pancreatobiliary applications [52]. The diameter of the fiber can be as small as 300 μm. Such a small diameter fiber can be used to image the pancreatic duct, bile duct, and be inserted through the fine-needle system currently used for performing EUS-guided FNA (nCLE). An ERCP is often performed to evaluate biliary and pancreatic strictures. Using a CholangioFlex confocal probe with Cellvizio (Mauna Kea Technologies, Paris, France), either the bile duct or the pancreatic duct can be further accessed. Early reports from several small series suggest excellent agreement between cytology/histopathology and focal endomicroscopy [53, 54]. Kahaleh and colleagues reported a Кappa coefficient of agreement of 0.8 between cyto/histopathology and pCLE in 15/16 cases (p = 0.0001). Furthermore, the clinical impact of the pCLE was such that 4 patients underwent a Whipple rather than a total pancreatectomy. Giovannini and colleagues found specific and reproducible patterns that reliably distinguished malignant from benign strictures using intraductal confocal microscopy (IDCM; Fig. 3.6). The presence of irregular vessels, large black bands, and black clumps seen on IDCM predicted neoplasia with a sensitivity of 83 %, specificity of 75 %, and accuracy of 86 % in 33 patients.
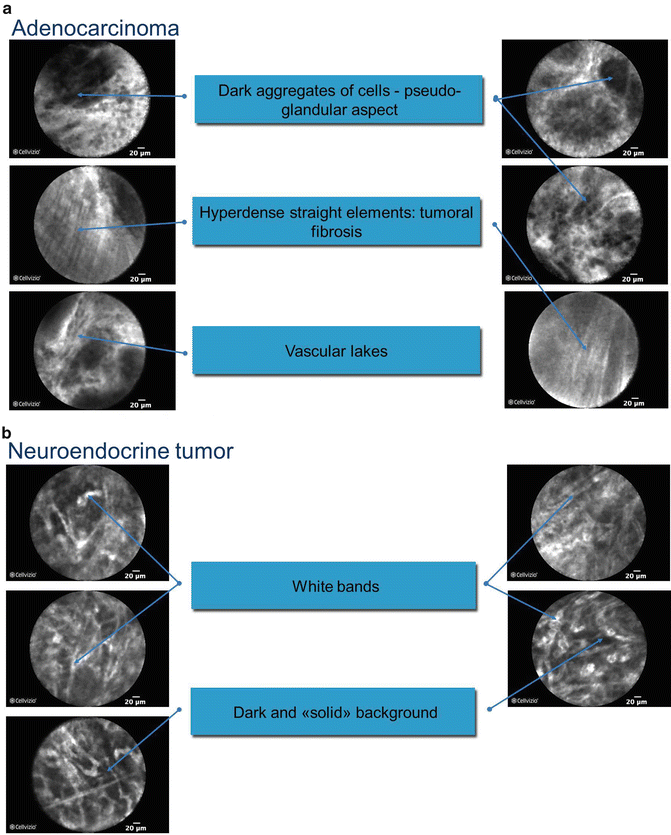

Fig. 3.6
(a) confocal laser endomicroscopy of pancreatic adenocarcinoma and (b) pancreatic neuroendocrine tumor (Images courtesy of Dr. Mark Giovannini)
Further advancement and refinement using pCLE technology has led to the development of a prototype new confocal miniprobe that is flexible and small enough to be introduced through either a standard 19-gauge or 22-gauge puncture needle currently used for EUS-FNA (nCLE) (Mauna Kea Technologies, Paris, France). Becker and colleagues showed that it was technically feasible to obtain in vivo histology in a porcine model using nCLE through a 22-gauge needle (Fig. 3.7) [55]. Konda et al. have taken this concept, model, and needle design to evaluate pancreatic lesions in humans [56, 57]. They found that an nCLE was technically feasible in 17 of 18 cases (2 solid lesions) using a 19-gauge needle with moderate to good imaging qualities (1-pancreatic endocrine tumor; 1-adenocarcinoma) [56]. Two patients suffered mild pancreatitis in cystic lesions, perhaps due to the larger needle size used. Konda et al. performed a subsequent, pilot in vivo nCLE multicenter study in the Pancreas with Endosonography of Cystic Tumors (INSPECT) [57]. They found that the presence of epithelial villous structures based on nCLE was associated with pancreatic cystic neoplasms (p = 0.004) and with a sensitivity of 59 %, specificity of 100 %, PPV of 100 %, and NPV of 50 %. The overall complication rate was 9 % with three cases of intracystic bleeding.
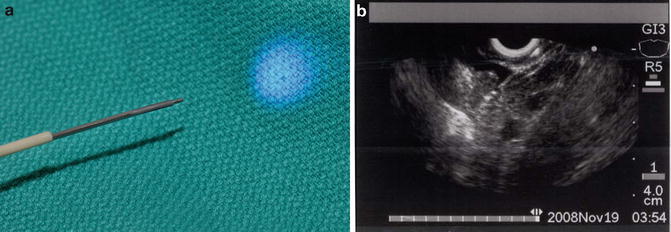

Fig. 3.7
(a) confocal probe through an EUS-FNA needle (nCLE) and (b) EUS image nCLE in a pig liver (Images courtesy of Dr. Michael Wallace)
EUS-Guided Cholangiography Biliary Drainage
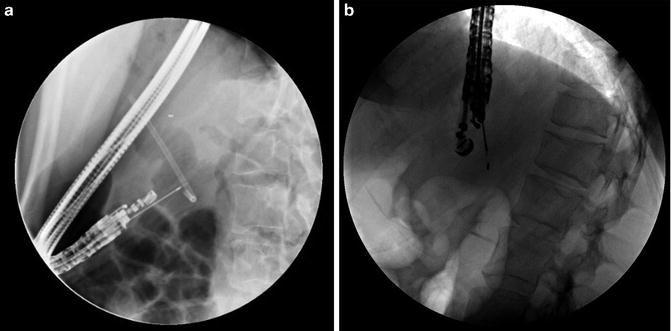
ERCP remains the procedure of choice for palliation of malignant biliary obstruction. Although technical success for ERCP hovers near 95 %, some caveats and challenges exist. Biliary drainage can elude even the most skilled biliary endoscopists in patients with variant anatomy, ampullary pathology, and/or malignant luminal obstruction. Traditionally, the drainage options when the papilla is not accessible include either percutaneous or surgical drainage [58–60]. The morbidity associated with these non-endoscopic approaches is high, especially in patients with advanced malignancy. EUS-guided biliary drainage (EGBD) provides an alternative for accessing the bile duct when transpapillary biliary cannulation fails (Fig. 3.8.) [61–64]. What makes it particularly attractive is that it can be performed in the same session as the originally failed ERCP without further delay. In addition, it provides immediate internal biliary drainage without the need for external drains. The first description of diagnostic EUS-guided cholangiography was published in 1996 by Wiersema et al. [65]. Subsequently in 2006, Giovannini et al. performed a palliative hepaticogastrostomy under EUS guidance in a patient with inoperable hepatic hilar obstruction [66]. EGBD can be achieved by one of two routes: (1) EUS-guided intrahepatic bile duct drainage, where the bile duct is punctured from a transesophageal, transgastric, or transjejunal approach, and (2) EUS-guided extrahepatic bile duct drainage, where the common bile duct is punctured from a transduodenal or a transgastric approach [67]. EUS-guided access and drainage can be either transluminal (endoscopic choledochoduodenostomy) or transpapillary (rendezvous) procedure. Over the past decade, several therapeutic modifications of EGBD have been developed. Both rendezvous and transluminal techniques seem to be equally effective and safe [62]. Based on the current literature, the cumulative success rate is 84–93 %, regardless of the approach, with an overall complication rate of 16–35 % [68]. Khasab et al. compared EGBD to percutaneous drainage (PTBD) in patients with malignant distal biliary obstruction who failed ERCP. Although technical success was higher in the PTBD group (100 % vs. 86.4 %, p = 0.007), clinical success, stent patency, and survival were equivalent in both groups. The PTBD group, however, was also associated with a higher adverse event rate and higher costs (>2 times) mostly due to a greater number of interventions [69]. Based on these limited, early, comparative trials, EGBD appears to allow an alternative technical approach for relieving biliary obstruction in select centers and with great caution.
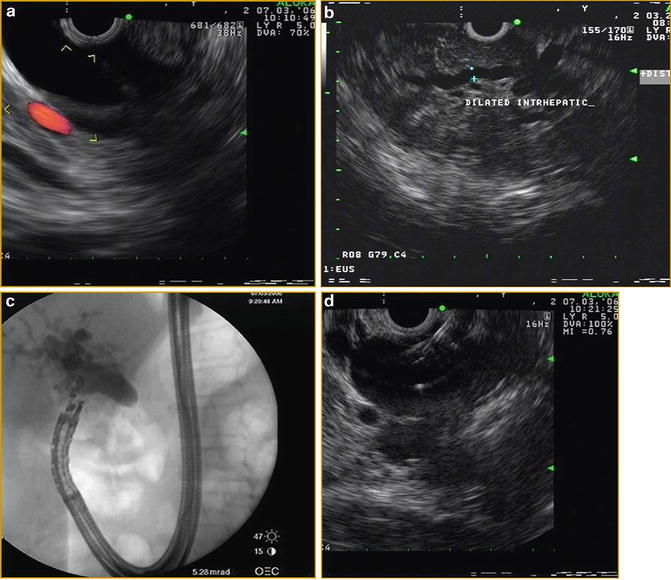
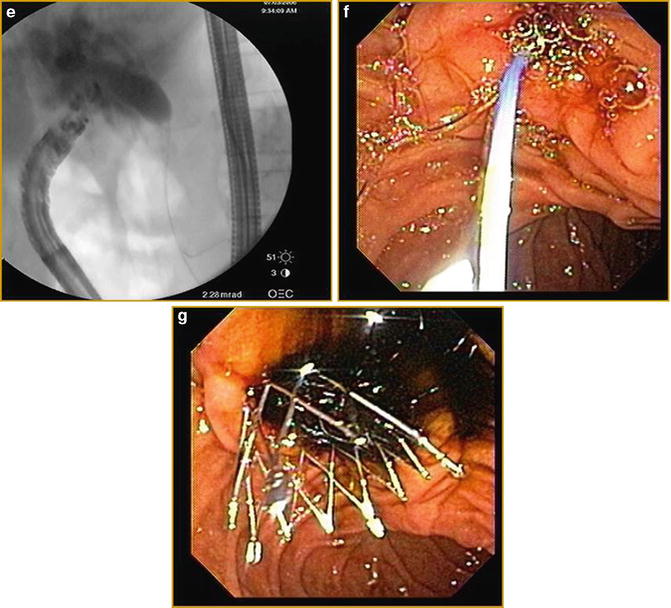


Fig. 3.8
Linear EUS images demonstrating EUS-guided cholangiography and biliary drainage after unsuccessful ERCP (a) EUS image of an approximately 2.0 cm, dilated CBD (b) EUS image of dilated intrahepatic ducts (c) fluoroscopic image of EUS-guided puncture of CBD and contrast injection (d) Puncture of CBD with a 19-g EUS needle (e) fluoroscopic image of guidewire placement via a 19-g EUS needle and antegrade passage of wire through the (f) papilla (g) deployment of metal biliary stent
EUS-Guided Fiducial Placement
In recent years, the development of image-guided radiation technique (IGRT) has allowed the delivery of precisely aimed radiation beams to tumors with great accuracy, thereby minimizing damage to the surrounding organs [70]. This is achieved by implantation of fiducials (cylindrical gold seeds) into the target lesion in order to target and track the location of tumor in real time (Fig. 3.9). The standard fiducials measure 3–5 mm in length and 0.8–1.2 mm in diameter. The new smaller, longer fiducial markers are 10 mm in length and 0.35 mm in diameter. To enable appropriate fiducial tracking by the CyberKnife system (Accuracy, Sunnyvale, Calif), it is recommended to place fiducials with “ideal fiducial geometry,” i.e., at least 3 fiducials with a minimum interfiducial distance >2 cm, minimum interfiducial angle >15°, and non-collinear placement in the imaging plane [71]. Traditionally, fiducial placement has been attempted either intraoperatively or percutaneously by interventional radiology under CT guidance. In the past few years, EUS has been increasingly used for fiducial placement in patients with inoperable pancreatic cancer [72–74]. With real-time visualization, Doppler imaging capability and the ability to access deeper structures within the GI tract, fiducial delivery can be successfully achieved using a 19-gauge or a 22-gauge needle. In a prospective study by Sanders et al., 51 patients with locally advanced or recurrent pancreatic cancer underwent EUS-guided placement of 0.8 × 5.0 mm fiducials using a 19-gauge needle [73]. Successful placement was achieved in 90 % of patients, with 91 % of patients successfully completing stereotactic body radiotherapy. There was 1 complication of mild pancreatitis occurring in a patient undergoing simultaneous placement of fiducials and celiac plexus neurolysis (CPN) for intractable abdominal pain. 91 % of patients successfully completed stereotactic body radiotherapy. Fiducial migration rate was low (7 %) and no migration-related complications were reported. Technical failures were seen in 4 patients (8 %) with recurrent cancer after pancreaticoduodenectomy.