Risk factors for CRC in chronic colitis
Extensive colitis [13]
Colon stricture [19]
Inflammatory pseudopolyps [19]
Extent of colitis can be divided into disease extending proximal to the hepatic flexure, which is associated with a higher risk of CRC; disease to the splenic flexure (left-side) that carries an intermediate risk; and proctitis or proctosigmoiditis that carries a low risk [13]. It is important to note that the extent of colitis is defined either macroscopically or microscopically, whichever reveals the furthest extent of inflammation. Mathy et al. demonstrated that neoplastic lesions can arise in areas of microscopic colitis that are otherwise grossly normal [27]. Whether early age of onset increases the risk for CRC, exclusive of an association with a longer duration of disease, remains controversial [3, 4, 13]. Increased risk of CRC with a concomitant diagnosis of PSC is clear [28] with estimated risks of 33 % at 20 years, and 40 % at 30 years after the diagnosis of UC [15]. A family history of sporadic CRC in a first-degree relative carries a twofold higher risk of CRC when compared to IBD patients without a family history of CRC [16, 17, 20]. Studies have confirmed that both histologic inflammation and endoscopic evidence of inflammation (i.e., presence of pseudopolyps, strictures, backwash ileitis, etc.) are independently associated with an increased risk of CRC [18–21, 29, 30]. Lastly, the presence of neoplastic change or dysplasia found during screening or surveillance colonoscopy is one of the strongest risk factors for CRC and is discussed in detail later.
Implications and Features of Dysplasia
Classification of Dysplasia
In general, dysplasia itself is known to be associated with an increased risk of CRC [24, 31], and often leads to a recommendation for prophylactic colectomy. To understand the endoscopic findings and surveillance strategies one must understand the classifications of dysplasia. Dysplasia is histologically defined as neoplastic alteration of the epithelium without invasion into the lamina propria, also termed intraepithelial neoplasia [31]. Traditionally the microscopic classification of dysplasia is divided into negative for dysplasia, indefinite for dysplasia, and positive for dysplasia [31]. Indefinite for dysplasia refers to changes in the epithelium that cannot be classified definitively as positive or negative for dysplasia either due to the confounding effects of inflammation and regeneration or to technical factors that impede their pathologic interpretation [32]. Dysplasia is further classified into LGD (also referred to as low-grade intraepithelial or noninvasive neoplasia), HGD (also referred to as high-grade intraepithelial or noninvasive neoplasia) or invasive cancer [31, 33]. It is important that histologic diagnoses of dysplasia be confirmed by a second expert gastrointestinal pathologist.
Grossly, most dysplastic lesions are endoscopically visible and their appearance in chronic colitis is heterogeneous, but is usually described as flat or elevated [34]. Flat dysplasia is the same as invisible dysplasia found on random nontargeted biopsies, and elevated lesions can be described as polypoid, plaque-like, slightly raised (flat), unifocal, or multifocal [35]. The growing use of the term “flat colon polyps” in non-colitic colons, which are now better visible through improved endoscopic imaging, can lead to confusion with flat LGD. Endoscopists must be clear whether the dysplasia was macroscopically visible (elevated) or invisible (flat). Raised dysplastic lesions discovered in areas of macroscopic or microscopic inflammation or prior inflammation are also referred to as dysplasia-associated lesion or mass (DALM) [36]. DALMs can then be further characterized into lesions that endoscopically appear to be consistent with sporadic adenomas, called “adenoma-like DALMs” or those that do not, called “nonadenoma-like DALMs.” If a well-circumscribed polyp with dysplasia is found outside the area of inflammation it is commonly referred to as a sporadic adenoma (Fig. 16.1a). If a similar appearing lesion is found within inflammation it is referred to as an adenoma-like DALM (Fig. 16.1b). Nonadenoma-like DALMs found in areas of inflammation typically have irregular and indistinct margins [36] (Fig. 16.1c). This terminology can be confusing since there might not be a difference between a sporadic polyp and an adenoma-like polyp when discovered in colitic mucosa; and when present in inflamed irregular mucosa it can be challenging to distinguish from a nonadenoma-like DALM [37]. These distinctions carry important prognostic implications with different management outcomes summarized in Table 16.2.
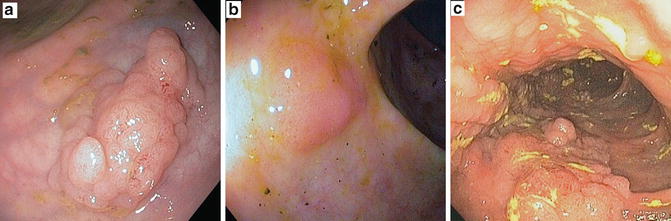

Fig. 16.1
Representative images of raised lesions seen on surveillance colonoscopy in chronic colitis. (a) An irregular sessile polyp in a part of the colon without inflammation (sporadic polyp) with clear defined borders that is amenable to complete resection. (b) A well-defined small sessile polyp was identified in a region of minimally active chronic colitis (adenoma-like DALM). This should be treated like a sporadic adenoma with goals of complete resection taking margins and biopsies from the surrounding basal mucosa. (c) An irregular, broad, nodular polyp (nonadenoma-like DALM) without clear defined borders was found in an area of chronic colitis. Surrounding mucosa was also positive for dysplasia. This was not endoscopically resectable. (Fig. 16.1c is courtesy of John F. Valentine, MD; University of Utah, Salt Lake City, UT.)
Table 16.2
Characteristics of dysplasia in chronic colitis
Dysplasia types | Endoscopic appearance | Initial management |
|---|---|---|
Sporadic polyp | A typical dysplastic or hyperplastic polyp with classic features of well-circumscribed borders outside the area of chronic inflammation | Routine polypectomy; if it is uncertain that chronic microscopic inflammatory changes are involved then obtain separate biopsies of the base and surrounding mucosa |
Adenoma-like DALM | Similar to a sporadic polyp but within the area of inflammation. These can be sessile, pedunculated, and slightly raised but retain well-circumscribed borders | Polypectomy with goal of snare resection that includes margins; in a separate specimen jar place random biopsies of base and surrounding mucosa |
Nonadenoma-like DALM | A slightly raised to visibly raised polyp that can be carpeting or nodular and typically irregular without distinct borders | Colectomy |
Flat HGD | Invisible random biopsy | Colectomy |
Flat LGD | Invisible random biopsy | Increased surveillance or colectomy |
Dysplasia and Risk of CRC
Unfortunately, carcinogenesis in IBD may not follow a progressive pattern from low-grade dysplasia (LGD), through high-grade dysplasia (HGD), to cancer [30]. Ullman et al. showed that cancer can arise in patients without prior dysplasia, without progression from LGD to HGD [23]. In fact even the diagnosis of indefinite for dysplasia carries risk of advanced neoplasia in 9 % of patients [5, 38].
The degree of risk for associated or synchronous CRC or HGD in patients with flat LGD is varied with studies reporting rates of 0–28 % [23, 24, 39, 40]. A recent meta-analysis examining 20 studies with 447 cases of flat LGD reported that the positive predictive value of flat LGD was 22 % for synchronous CRC and 36 % for synchronous HGD and CRC [41]. Further raising controversy, two large cohort studies from Europe found only a 2–10 % frequency of advanced pathology over a 10-year period in patients with flat LGD [25, 26]. The debate will continue regarding the degree of risk for synchronous or future advanced neoplasia, but there clearly seems to be a risk. There is a better consensus with the risk of CRC from flat HGD with historical cohort studies and reviews indicating a risk of synchronous or metachronous CRC in up to 42–67 % of patients [3, 22, 24, 42].
There is also agreement on the risk of advanced neoplasia after finding a raised lesion with dysplasia, although it depends on whether it is found in an area of IBD or not (summarized in Table 16.2). Nonadenoma-like DALMs carry a risk for synchronous occurrence of CRC as high as 43–58 % and thus are referred for total colectomy [22, 36]. Adenoma-like DALMs carry a negligible risk of associated CRC similar to that of sporadic polyps [43, 44]. Additionally the risk of CRC does not change if the adenoma-like DALM is inside or outside the area of inflammation [45]. The key in this situation is to make sure the polyp in the area of inflammation is actually an adenoma-like DALM.
Surveillance Strategies
Efficacy of Surveillance Colonoscopy
Surveillance strategies are aimed at early detection and mortality reduction from CRC in IBD. Evidence for the effectiveness of surveillance programs reducing morbidity and mortality from CRC is not strong due to the limitations of currently available studies, reviewed in Ahmadi et al. [46]. A recent Cochrane systematic review reported that there was no strong evidence that surveillance colonoscopy prolonged survival in people with chronic colitis, but there was evidence that suggested CRC found at an earlier stage led to a better prognosis [47]. However, a recent retrospective study of 149 subjects from the Netherlands identified a significant survival benefit for patients diagnosed with CRC during surveillance colonoscopy, with a 5-year overall mortality of 0 % for people undergoing surveillance versus 36 % for those not undergoing surveillance [48].
Current Guidelines
Although there is consensus among international gastroenterology societies that persons with chronic ulcerative colitis and chronic Crohn’s colitis undergo surveillance colonoscopy, differences exist on when to start screening, the interval of surveillance, and even the method of surveillance. These differences among American, British, European and Asian societies are summarized in Table 16.3 [49–57].
Table 16.3
Comparison of screening and surveillance guidelines in IBD
Recommendation | American | British | European | Asian |
|---|---|---|---|---|
Screening initiation | ||||
Extensive colitis | 8–10 year after symptoms | 10 year after symptoms in all patients to assess disease extent | 8 years after symptoms | 8–10 year |
Left-sided colitis | 15 years after symptoms | 8 years after symptoms | 12–15 years | |
Proctitis | Standard CRC guidelines | Standard CRC guidelines | Standard CRC guidelines | |
PSC | At diagnosis | At diagnosis | At diagnosis | No clear recommendation |
Ileal pouch | No clear recommendation | No clear recommendation | No clear recommendation | No clear recommendation |
Colonoscopic methods | ||||
Random biopsies | Yes (≥33) | Only if chromo n/a (≥33) | Only if chromo n/a (≥33) | No clear recommendation |
Chromoendoscopy | Supportive but NR | Recommended | Recommended | |
Follow-up surveillance | Every 1–3 years Consider annually for disease greater than 20 yrs No clear recommendation for surveillance in the ileal pouch | Stratified by risk Low risk: every 5 years Intermediate: every 3 years High risk: annually If surveillance desired in pouch then annually in high risk and every 5 years in low risk | Stratified by risk Low risk: every 3–4 years High risk: every 1–2 years No clear recommendation for surveillance in the ileal pouch | Every 1–2 years in Japan |
Current American guidelines do not stratify patients according to risk exclusive of a diagnosis of PSC and disease extent. However, evidence supports that risk stratification to determine surveillance intervals may be a cost-effective approach, and is currently followed by British and European guidelines. European risk stratification is defined as a scoring system, with one point for each risk factor (pancolitis, endoscopic and/or histological inflammation, pseudopolyps, and family history of CRC) with low-risk patient scores of 0–2 and high-risk scores of 3–4 [55]. British risk stratification is defined as: low risk (no endoscopic/histological inflammation or left-sided colitis or Crohn’s disease colitis affecting <50 % surface area of the colon); intermediate risk (mild endoscopic/histological active inflammation or presence of post-inflammatory polyps or family history of CRC in a first-degree relative aged 50 years or over); and high risk (moderate or severe endoscopic/histological active inflammation or stricture within past 5 years or confirmed dysplasia within past 5 years in a patient who declines surgery or PSC/post-orthotopic liver transplant for PSC or family history of CRC in a first-degree relative aged <50 years [53].
Limitation of Surveillance Colonoscopy
Colonoscopic screening and surveillance for CRC in chronic colitis is widely accepted, and even though guidelines evolve, several limitations remain. First, a high sampling error exists, with <1 % of the entire mucosal colonic surface area sampled if 32 biopsies are obtained [30]. Despite growing evidence that most dysplasia in chronic colitis is visible, approximately one-third is flat invisible dysplasia requiring optimal sampling or improved endoscopic techniques [34]. Current guidelines are based off the estimate that 33 biopsies provide a 90 % probability of dysplasia detection [58].
Next, adherence to guidelines by patients and providers are paramount to screening success. A large cohort study from California reported that less than one-third of at-risk patients underwent surveillance colonoscopy in a two-year period. These low rates were also reported by a nested study from the French CESAME cohort that showed a reduced rate (54 %) of surveillance in at-risk subjects with the lowest rates of surveillance in subjects with Crohn’s colitis and those not cared for at specialized centers [59]. Studies have also demonstrated that providers often stray from guidelines and take less than 33 biopsies [60–62].
Making an accurate histologic diagnosis is also vital for proper surveillance outcomes. In addition to the reactive atypia that may confound pathologic interpretation, there is a large degree of interobserver disagreement among pathologists when distinguishing between LGD and HGD [63, 64]. As part of the guidelines it is recommended that all specimens concerning for dysplasia be confirmed by a second gastrointestinal pathologist [65].
Finally, as the guidelines evolve to recommend resection of raised dysplastic lesions as an alternative to colectomy, the adequacy of resection is increasingly important [66]. However, anatomical interference can limit the effectiveness of proper identification and complete resection of lesions. Three circumstances encountered during surveillance colonoscopy need to be addressed further: pseudopolyps, UC-associated strictures, and Crohn’s-associated colonic strictures. In the setting of multiple or diffuse pseudopolyps, identification of suspicious lesions can be a daunting task. A case–control study by Velayos et al. demonstrated that presence of pseudopolyps was associated with a 2.5-fold increased risk in the development of CRC [20]. Strictures in UC should arouse a high suspicion of CRC as studies have shown that a quarter of them are malignant [67]. In the setting of colonic Crohn’s strictures, the risk of underlying malignancy is not as high as in UC, but is still present. A review of 175 Crohn’s colon strictures revealed a 6.8 % frequency of colon cancer after 20 years of disease duration [68]. Therefore surgical resection should be considered if one is unable to fully evaluate the stricture or proximal colon [68, 69].
Surveillance Colonoscopy with Random Biopsies
To reduce the risk of CRC or CRC-related mortality it is recommended that patients with UC extending beyond the rectum and extensive Crohn’s colitis involving more than a third of the colon undergo surveillance colonoscopy following the timelines suggested in Table 16.3. Since dysplasia in chronic inflammation or healed colitis can be invisible (flat), only slightly raised, or difficult to visualize in a field of active inflammation, current guidelines in the U.S. continue to recommend obtaining random, nontargeted biopsies using white light endoscopy (WLE). This section of the chapter will review the details and evidence that will help optimize WLE endoscopy technique in colitis surveillance, including procedural quality, biopsy techniques and specialized situations.
To improve quality of dysplasia detection during WLE it is recommended that the bowel preparation be of good quality, and that the disease be in clinical remission if possible. Although evidence does not exist for the influence of bowel preparation on dysplasia detection in colitis, it has been reported that patients with colitis have worse prep outcomes [70]. Good bowel preparations will aid in the ability to visualize the heterogenous lesions in IBD. Similarly, the presence of active inflammation may interfere with the ability to visually recognize the subtle lesions commonly found in chronic colitis, and histologically differentiate reactive atypia from indefinite for dysplasia or LGD [71]. Thus current guidelines recommend performing surveillance when disease is quiescent if possible [49]. Equipment may also make a difference in the quality of dysplasia detection. High definition endoscopic video imaging is widely available and when examined in a retrospective cohort study of colitis surveillance it performed better than standard definition WLE with an adjusted prevalence ratio of detecting any dysplastic lesion of 2.21 (95 % CI, 1.09–4.45) [72]. Variation also exists among biopsy forceps and experts have recommended that jumbo forceps can be used to obtain random surveillance biopsies to increase tissue sampling [73]. Two recent single center studies have demonstrated increased tissue volume using jumbo forceps during random colon biopsies [74, 75]. However, guidelines do not recommend a forceps type since head-to-head studies examining dysplasia detection do not exist.
Traditionally, random biopsies have been performed utilizing a four-quadrant technique sampling the entire colon every 10 cm in addition to biopsies of suspicious lesions. Thus in an 80–100 cm colon this yields a minimum of 32–40 biopsy specimens. It is important to note that this accounts for <1 % of the total colonic surface area, resulting in the potential for false-negative results [30]. As previously discussed these numbers are required to detect dysplasia with 90 % confidence [58]. A recent mathematical modeling study reduced this probability, reporting that 32 random biopsies provide only 80 % confidence that dysplasia involving greater than or equal to 5 % of the colon can be detected [76]. These limited data would suggest that many more random biopsies are required to detect dysplasia. Some experts recommend sampling every 5 cm in the rectosigmoid colon, given the higher frequency of dysplasia in this area. A retrospective study from Mount Sinai reported that the rectosigmoid colon demonstrated the highest percentage of biopsies positive for any neoplastic lesion and advanced neoplasia [77]. Finally, similar to colon cancer screening in noncolitic patients, a slower withdrawal time will increase dysplasia detection rates [78].
Ideally, multi-bite forceps should be used with no more than two mucosal bites taken in a single pass. This technique is demonstrated in Video 16.1. Experts suggest no more than eight biopsies should be placed in separate containers and labeled by location. Locations are best separated into (1) cecum/ascending colon, (2) transverse colon, (3) descending colon, (4) sigmoid colon, and (5) rectum. This time consuming and iterative process could distract the endoscopist from properly scanning the mucosa for subtle irregular lesions. The endoscopist should remain vigilant and utilize all qualified endoscopy staff assisting in the procedure to help monitor for suspicious lesions, which should be sampled separately. Raised lesions that resemble sporadic adenomas or adenoma-like DALMs should be completely resected, with separate biopsies of the surrounding tissue sampled as well. Further details in the management of dysplasia are provided later in this chapter.
There are no formal recommendations regarding surveillance biopsy techniques in the setting of inflammatory pseudopolyps. Pseudopolyps are raised polypoid lesions developed through recurrent epithelial regeneration and can be distinguished, by experienced endoscopists, from sporadic adenomas or raised dysplasia through their pit patterns on high-definition imaging utilizing magnification and contrast [79]. However in fields of active inflammation or with routine WLE this distinction is less clear. Additionally, when pseudopolyps are numerous they may obscure the visibility of adenomas or DALMS [19, 20]. Through communication with experts these lesions are typically targeted during random surveillance biopsy in an attempt to minimize the risk of missing dysplasia in irregular mucosal surfaces. It is important to communicate these limitations with the patients that have numerous colonic pseudopolyps so future surveillance risks can be continued through a joint decision-making process.
Endoscopists may also encounter patients who have had a subtotal colectomy and now are left with a closed rectal pouch. This is mostly encountered in Crohn’s colitis. The remaining rectal pouch should be surveyed following pre-colectomy risk factors to guide surveillance intervals. A small retrospective case control study from the Netherlands confirmed that the risk of CRC persists in closed rectal stumps and was seen mainly in patients with PSC or patients with greater than 8 years of disease [80].
Overall, the random biopsy surveillance technique is a laborious and costly process with historically reduced adherence by patients and providers. Thus a paradigm shift is underway toward a more focused and targeted biopsy approach through visual contrast imaging techniques. However, if specialized training or resources are not available then random biopsies continue to be an acceptable method for dysplasia surveillance in patients with chronic UC or Crohn’s colitis. But should we completely abandon random surveillance biopsies? If targeted biopsies can identify 60–88 % of neoplasia [34, 81–83], it could be extrapolated to a potential miss rate of 12–40 % of neoplastic lesions. Although current data might suggest that targeted biopsies are better at identifying neoplasia as compare to random biopsies, endoscopists might want to consider doing both to optimize detection rates. At present, a prospective randomized controlled trial is underway in Japan, comparing targeted biopsy versus random biopsy approaches [84]. This will hopefully provide better evidence to optimize our current endoscopic protocols.
Management of Dysplasia and Approach to Polypectomy
If we consider the benefits of discovering raised and flat (invisible) dysplasia, the role of the endoscopist is to detect, describe, and biopsy or remove all endoscopically visible lesions; plus obtain biopsies from flat “normal” appearing mucosa. Then after rigorous histologic assessment of several biopsies for a single patient, a diagnosis is made that stratifies the patient into a path of continued surveillance or proper dysplasia management. Table 16.1 outlines the approach to managing dysplasia summarized from the three most current guidelines. In the instance that an “indefinite for dysplasia” diagnosis is made and confirmed, guidelines advise optimization of therapy and follow-up with a repeat colonoscopy in 3–12 months [49]. The greater the number of risk factors the sooner it should be repeated. As already discussed, the risk of synchronous and metachronous CRC after finding flat HGD and nonadenoma-like DALMs (with LGD or HGD) is significant and a colectomy is recommended. However, the elephant in the room is flat (invisible) low grade dysplasia. Consensus has not been reached on how to manage this finding due to its variable natural history in previous cohort studies.
Approach to Flat LGD
If flat or invisible LGD dysplasia is found on colonoscopy the management is not clear primarily because of conflicting data regarding the progression of flat LGD to CRC and the controversial existence of concurrent advanced neoplasia. Studies have revealed a LGD to HGD or CRC progression rate ranging anywhere from 2 to 50 % [22–26]. Therefore, it is important to discuss the risks and benefits of both aggressive surveillance (repeat surveillance colonoscopies every 3–6 months) and the option of a proctocolectomy with patients at the time LGD is identified. If the dysplasia is multifocal flat LGD (identified in different locations of the colon during the same colonoscopy) there may be an increased risk of progression to CRC and perhaps more a push toward colectomy. A prospective study from the University of Washington followed 42 patients with LGD and the rate of progression to advanced neoplasia over a 4-year follow-up period was low, but for those patients with four or more biopsies that contained LGD the risk of progression to advanced neoplasia was greatest, RR 5.8 (95 % CI, 1.29–26.04) [40]. The risk of progression to advanced neoplasia in the setting of recurrent flat LGD (also identified on the subsequent surveillance colonoscopy) is again less clear since sampling error might create a false negative. Thus for multifocal or recurrent flat LGD the Crohn’s and Colitis Foundation of America consensus strongly recommends a prophylactic total proctocolectomy [69].
Endoscopic Approach to Raised Lesions
Management recommendations for raised or visible lesions that contain dysplasia on histologic examination (DALMs) depend on their endoscopic appearance and degree of removal. Raised lesions that resemble sporadic adenomas (adenoma-like DALM) should be completely resected with snare polypectomy technique with biopsies obtained from the adjacent flat mucosa surrounding the polyp base, and placed in a separate container, demonstrated in Video 16.2. If the flat adjacent mucosa contains dysplasia, then these lesions should be treated as nonadenoma-like DALMs and colectomy should be recommended. Otherwise, if there is no dysplasia in the adjacent or distant tissue, a repeat colonoscopy should be performed within 6 months [49]. Then, if no further dysplasia is identified, surveillance can be resumed every 1–2 years. A long-term follow-up study of 34 patients revealed no significant difference in future polyp formation between UC patients with an adenoma-like DALM (62.5 %), UC patients with a sporadic adenoma (50 %), and non-UC sporadic adenomas (49 %) [85]. Two other retrospective cohort studies revealed a 0–2 % risk of developing advanced neoplasia in follow-up after polypectomy [37, 86]. The endoscopic management of adenoma-like DALMs is appropriate and can be safely monitored by surveillance colonoscopy. On the other hand, if the lesion is recognized as a nonadenoma-like DALM, there is a significant risk of concurrent or metachronous CRC (described previously), thus a colectomy should be recommended [37, 85].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








