Fig. 25.1
Preparation of ex vivo porcine model. (a) Incision about 10 cm along the greater curvature with a scalpel; (b) Open porcine stomach greater curvature; (c) Gauze is applied to dry the tissue after profuse washing with water; (d) Tattooing artificial lesion with China ink; (e) Porcine stomach sutured with vicryl; (f) Plastic box with cadaveric model
Preparation of the Ex Vivo Colonic Model
An ex vivo colonic porcine model was originally developed in the Endoscopy Unit of the Tokyo NCC for practicing ESD. This model, which is similar to the one developed for gastric ESD, has been successfully reproduced by others [48]. It is a simple model to prepare, low in cost, and, in this authors’ experience, helpful for training colorectal ESD. Japanese experts also find the esophagus appropriate for training colorectal ESD. Fatty tissue is rather abundant in the porcine colon and rectum, which can hamper viewing the operation field because the lens is repeatedly stained by fat.
To assemble the model, a plastic box is used to mimic the abdomen, with a hole on one end to insert the endoscope. It is preferable to use a disposable laparoscopic port to dock with a plastic tube to simulate the anus, which helps to maintain a hermetic environment during the procedure. The distal end of the colon is attached to prevent air leakage and the other end is securely clamped to the plastic tube that houses the laparoscopic port.
Another way to build the ex vivo colon model is with a polyphony sheet that dynamically simulates the anatomy of the colon. The colon is inserted into the limited space and fixed in the same way as with model described above. A section of the colon can be wrapped in foil to increase electrical conductivity. The advantage of the model is its resemblance to human anatomy due to the presence of angles. As mentioned above, artificial lesions can be created by tattoos.
Yoshida et al. evaluated isolated bovine and porcine colon models, including blood flow to extend the training to hemostasis and make the procedure more realistic [49]. Research is needed to determine the best model for colorectal ESD training in this complex location.
Preparation of the Live Model
The in vivo porcine model has also proven suitable for endoscopic ESD training. The live model has the advantage of being more realistic because of the presence of peristalsis, intraluminal secretions, and bleeding, the latter representing a possible complication (although in the experience of the authors, bleeding during ESD in living models is less common and profuse than in humans). Another advantage of the live model is the possibility of assessing results in terms of survival rates.
We agree with the widely held view that in the first stage, basic ESD maneuvers and strategies can and should be learned with the ex vivo model and that there is no justification for using live pigs at that stage. Use of live pigs is more costly, requires more complex preparation and technical assistance, and, most importantly, would not be ethical.
The common domestic pig (Sus Scrofa Domestica) is used for training. It is generally recommended to use young pigs that weigh 30–40 kg. Notably, there is a small cul de sac at the caudal end of the pig pharynx called the pharyngeal pouch that can hinder endoscopic intubation.
Animals should be sedated and monitored by a veterinarian for any possible complications. The veterinarian must also be responsible for euthanizing the animals. Two strategies have been proposed for sedating pigs in in vivo models: (1) induction and maintenance with ketamine and midazolam or propofol, (2) intramuscular premedication with ketamine and maintenance with propofol and inhalation anesthesia (halothane, isoflurane or sevoflurane). Intravenous medication is administered by placing an easily accessible venous catheter in the ear. Occasionally, hyoscine butyl bromide may be necessary to decrease gastrointestinal motility.
The live model can be used for practicing not only gastric ESD but also with the esophagus and colon. In the latter case, one of the main limitations is the difficulty in cleaning the colon. Polyethylene glycol or sodium phosphate should be given orally for the preparation at least 2 days before the procedure. Bowel preparation can be completed by enemas after anesthetizing the animal. It is also important that the animal be kept away from food or objects that can be ingested. It is not unusual to find stomachs packed with ingested contents in live porcine models used in hands-on courses, which can undo the value of the activity. Given this, it is advisable to keep the pig on a liquid diet for 24 h and a complete fast for 8 h prior to the procedure.
Other limitations of the live model for practicing colonic ESD are: (1) the large amount of fat in the submucosal layer; (2) a thinner colon wall than that of humans; (3) differences in the structure of the pig colon from the human colon; and (4) the lack of abdominal fixations in the proximal colon (the large intestine only resembles that of humans in the rectosigmoid segment). These differences may limit the degree practice results in overcoming technical difficulties and the risk of making perforations.
The Gap Between Animal Models and Real Patients: The Western Perspective About Recommendations For ESD With Humans
The first approach to ESD procedures on real patients is always challenging. Even if training with animal models is exhaustive and well planned, there is a gap between ESD with animal models and with humans. In fact, in the animal models, only normal mucosa is resected, while with humans we are treating lesions. The most important factors in approaching the first human ESD with confidence is to have had sufficient training with animal models for both ESD and handling complications, and to have performed such procedures with the same seriousness and concentration as with procedures with human patients. Although ESD procedures with animals are more relaxed and can even be undertaken with fun, we should remember that human ESD will not be so much fun when difficulties arise. As well, ESD should be elected when there are appropriate indications, because of which it is advisable to obtain expert (Japanese) consultation before electing to perform ESD. Experts confirm whether or not lesions show any signs of deep submucosal invasion, as well as providing guidance about the ESD strategy. Such advice can be invaluable during the procedure.
Another possibility with initial cases in the era of telemedicine is distance supervision via internet, ideally with high-quality transmission [50]. The first author attempted his first human ESD case with this form of supervision, provided by Dr. Oda and others at the National Cancer Center in Tokyo, which definitely was helpful.
The setting where the procedure is to be performed can be relevant. In Japan, ESD is almost always performed in the Endoscopy Unit. Recently the practice of sedating patients with propofol by non-anesthesiologists has been introduced in expert Japanese and Korean centers [5, 51]. We believe that in particular for upper GI ESD, having an anesthesiologist or a well-trained non-anesthesiologist present to handle sedation can be reassuring for the endoscopist, who can then concentrate exclusively on the endoscopic aspects of the procedure.
Continuous infusion of propofol and an opioid during gastric ESD is associated with a significantly higher rate of aspiration pneumonia than is the use of midazolam and intermittent propofol infusion (4.4 % vs. 1.5 %, P = 0.002) [53]. This is attributed to a decreased gag reflex with the former. On the other hand, general anesthesia with intubation is associated with significantly shorter procedural time for gastric ESD (nearly half, on average) than sedation with midazolam. As well, the complication rate is lower with anesthesia, although not significantly, and patient satisfaction is significantly higher. With these results, western endoscopists are recommended to perform upper ESD with general anesthesia, at least with the initial cases, until the procedure time can be reduced with experience. General anesthesia has the advantage of permitting the operator to work with less pressure, which can facilitate dealing with complications that may arise. The operator can feel as if they were working in the animal lab, where conditions are more relaxed, as opposed to the situation where the patient is only sedated, and especially if the operator is in charge of sedation.
Midazolam and pethidine or fentanyl are generally used in colorectal ESD in Japan. It is often necessary to change the patient’s position several times during colorectal ESD to take advantage of gravity and avoid liquid accumulating in the target area. Deep sedation with propofol has the theoretical disadvantage that it makes changing the patient’s position more complicated. However, in the authors’ experience, Western patients tolerate colorectal ESD much worse, including when performed by Japanese experts, with deep sedation with propofol than with midazolam and an opiate.
Another step from the lab to the patient is that all the details related to the configuration of the procedure room, patient position, type of sedation, and the availability of experienced assistants have to be taken into account before the procedure, and are likely to be different from those in the animal lab. Even the equipment (endoscope, accessories, electrosurgical generator) may be different from those for training with animal models, although ideally similar equipment is used in both settings. Planning the configuration of the operating room is critical to ensure smooth and efficient procedure.
If the operator is a gastroenterologist that is unfamiliar with the operating room, it can seem an unknown territory. In this case, it is advisable that the operator visit the area in advance and discussing the best configuration for the procedure with the personnel. It is also advisable to discuss the case with a surgical team before the procedure, in case surgery is needed during or after the ESD.
Upper GI ESD (Fig. 25.2)
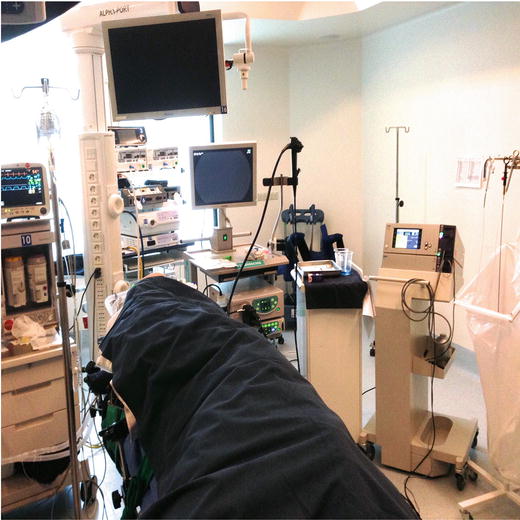
Fig. 25.2
Configuration of the operating room for upper ESD in a western center
Patient Position
The patient has to be placed on his/her left side as in a regular endoscopy, but it is advisable to use a soft mattress to protect the patient against scarring since the procedure can last several hours. The skin at all the pressure areas has to be protected and the extremities need to be secured to the patient’s body with soft pads so that they do not interfere with the procedure. The tracheal tube also has to be placed at the back of the patient’s mouth so that front of the mouth is clear and the operator can move their hands without interference. It is also important to use a short overtube, so that the endoscope can be easily removed and reinserted during the procedure.
Operation Room Configuration
A recommended configuration for the operating room is to place the endoscopy tower at the top end of the patient’s bed. This monitoring tower will be used by the operator and the assistant. However, if possible, a larger monitor should be placed behind the patient so that first the operator can work with it more comfortably. A small table should be located to the right of the endoscopy tower from the operator’s point of view on which are placed two 200–300 cc basins (one with the submucosal fluid cushion (SFC) solution and the other with saline) and three syringes (one of 20 ml for the SFC, another of 20 ml for saline, and finally one of 50 ml for a mixture of water and simethicone to wash the GI lumen). The electrosurgical generator is placed next to the table and is managed by the first assistant, who needs to have a complete knowledge of the energy source and how to change the settings whenever required. Needless to say, all the settings have to be selected and programmed into the electrosurgical unit before the procedure. Last but not least, an endoscopy rack is placed at the right of the energy source with all the devices ready to be used. This is very important, because during an ESD procedure it may be necessary to change endoknives quickly and frequently. Independent of any preferences among endoknives, it is highly recommended to equip the ESD rack with a high flow injection needle, coagulation forceps, and at least two different types of knives. Among the knives we are most familiar with, the most advisable for a first procedure are an insulated-tip knife and a needle knife.
Lower GI ESD
Things are somewhat different in case of lower GI ESD. Even for western endoscopists still in the learning process, it is advisable to perform colorectal ESD under deep sedation using an endoscopy bed rather than an operating room bed. Endoscopy beds are wider than most operating room beds and allow the colonoscope loop (if it is the long type that are most commonly used in western endoscopy units) to rest comfortably on the bed. This simple detail can be a great help in a difficult colonic ESD cases. Moreover, the fact that the patient is under deep sedation supervised by an anesthesiologist, most often with propofol, makes it possible to change the patient’s position with relative ease, as is often necessary with colonic ESD. Another advantage of performing colorectal ESD in the endoscopy unit is that it is likely that the patient can receive the agent for the bowel preparation according to a schedule that follows current recommendations (2–5 h before the examination), while patients sent to the surgical area are usually required to fast for at least 8 h. This results in poor preparation, representing a serious problem for the performance of ESD, including the treatment and prognosis of any complications, mainly perforations. Colonic ESD has the highest risk of perforation, so the surgical team must be familiar with the procedure should surgery be needed. Apart from patient position and the recommended bed, the configuration of the operation is the same as for gastric ESD.
Final Tips
A second assistant should ideally be next to the first to clean the devices when they are not being used. It is very useful to have a surgical blade and/or a toothbrush for this purpose because endoknives tend to accumulate debris that can interfere with their adequate functioning. The second assistant can also pass the operator other things needed during the procedure so that the operator can focus solely on the ESD.
Treatment of Complications as a Central Part of Training in ESD
During the ESD training process, developing the essential skills to successfully manage complications is of paramount importance. Perforation and bleeding are the main adverse events in ESD and the trainee must be aware and ready to handle them before attempting human cases [31, 54, 55]. As noted above, training with animal models should include dealing with ESD-related complications [40–56], initially with isolated organs, and after with live animals to develop skills in perforation closure and hemostasis of bleeding vessels.
Perforation
The trainee should take any opportunity after unintentional perforation during ESD to practice secure closure of the defect. If possible, the organ should be examined meticulously to confirm the quality of the closure (Fig. 25.3). Clipping is not always easy, in particular, with precise closure of target lesions in difficult locations. Skills in this area can make a substantial difference between a successful ESD and a frustrating failure with unpleasant results for the patient. However, ongoing training implies using many clips, which is not affordable for many centers. One alternative is the use of expired devices donated by manufacturing companies for training purposes.
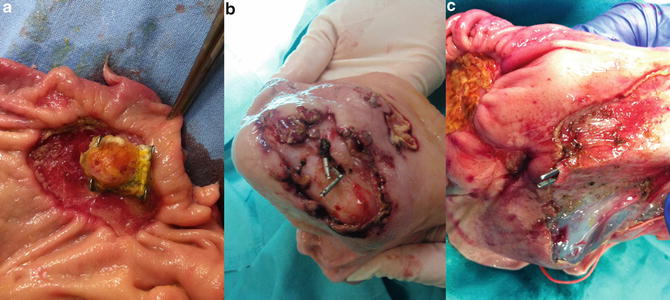

Fig. 25.3
Necropsy specimens of porcine stomach after ESD (interior view): (a) Closure of gastric perforation with OTSC clip; (b) Closure of gastric perforation with a Resolution clip®; (c) Demonstration of incomplete closure of perforation using surgical forceps
Bleeding
Bleeding is a common complication during ESD procedures. Although some authors have reported setting artificial vessels in isolated harvested organs, only live animals can realistically reproduce the bleeding experienced with patients [49].
The trainee should anticipate and avoid bleeding with preventive coagulation based on adequate identification of vessels in the submucosal level [57]. It has been suggested that the deeper dissection level in the submucosal layer is associated with fewer vessels and less procedural hemorrhaging [58]. There are more details on this in Chap. 16.
Post-ESD Stenosis
This complication is usually related to radical, full circumferential ESD, either in the esophagus, the stomach, or the rectum. In animal model training we can only experience this in survival studies, where the animal undergoes endoscopy 2–4 weeks after the initial ESD.
Summary
In summary, intensive training for the successful management of complications is key for the ESD apprentice. Animal models provide an excellent opportunity to develop the required skills to deal with complications associated with ESD, especially perforation and bleeding. In our western environment, only through continuous practice will we have a good chance to obtain full competence in ESD.
Is More Training Required for ESD?
Most experts in the area argue that ESD is the final step in the training for early detection and treatment of GI neoplasias. Detection is the most important issue, since lesions undetected by endoscopists remain treated until they become symptomatic, while lesions that are detected are treated, whether by endoscopy or surgery.
Training for advanced diagnostic endoscopy is beyond the scope of this book, but the most advanced and experienced centers in diagnosis of early lesions are in Asia, and, if possible, training there is highly desirable.
The importance of an adequate endoscopic diagnosis before ESD cannot be overstated. The operator should understand perfectly the type of lesion to be treated, as well as its location and extension. To this end, diagnostic endoscopy should ideally be performed by the same operator who will perform ESD. Especially in the cases of squamous esophageal and gastric cancers, it can be impossible to trace the margins of the lesions with only white light endoscopy, even with high definition endoscopes. Digital or Lugol’s iodine chromoendoscopy should be used for squamous lesions, while for flat-type early gastric cancers, indigo carmine, acetic acid-indigo carmine, or NBI with magnification can show the margins of most lesions. Although taking too many biopsies from lesions before ESD should be avoided to prevent the development of fibrosis, which can make ESD more challenging, biopsies should be taken to clarify the extension of the lesion and determine if there are synchronous lesions.
Technical Issues That May Help Western Endoscopists to Perform ESD
At least two technical issues should be considered by western endoscopists training in ESD. The first relates to performing more complex resections still using the snare, before moving to full ESD (transitional resections). Especially in the colon, small incision-assisted EMR is used for en bloc resection of lesions that cannot be easily trapped en bloc by the snare [59]. The technique consists of making a small slit with the tip of the snare in the normal mucosa at the oral margin (after injecting sufficient solution). The tip of the snare remains in the slit and the snare is opened, preventing slippage of the snare distally and keeping it in an adequate position. The snare is then closed slowly.
The second technique is called hybrid EMR and represents an early application of ESD [60, 61]. In this technique, after having achieved the circumferential resection with an ESD knife, a snare is applied to complete the resection. The method can be applied as rescue therapy when it is difficult to complete ESD more conventionally, but can also be performed as a planned procedure. It is currently applied mainly with colonic lesions and has proven useful for lesions up to 3 cm in diameter. The method is an excellent way for operators to gain confidence with the use of the knives on humans, and in fact, some Japanese ESD experts have described their experience with the two methods described above while learning colorectal ESD [59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








