Fig. 1.1
Endoscopic fluorescence imaging systems for bladder cancer. (a) Photodynamic diagnosis (PDD) composed of a blue-light source, specialized camera head and lens, and hexaminolevulinate as the contrast agent. (b) Confocal laser endomicroscopy (CLE) composed of a 2.6-mm fiberoptic imaging probe, fluorescein as the contrast agent, and the laser scanning unit
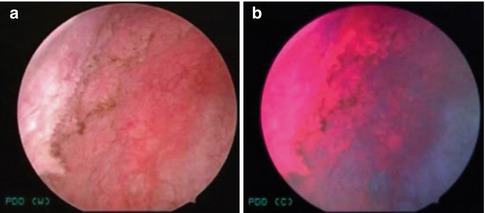
Fig. 1.2
PDD of bladder cancer. (a) White-light image of a large flat lesion at the right bladder wall showing indistinct borders and (b) the corresponding PDD image. The region was confirmed to be carcinoma in situ (CIS) (From Hsu et al. [27], with permission)
Integration in the OR environment is overall straightforward. HAL is solubilized and instilled intravesically by either the surgical team or nursing staff via a catheter 1–3 h prior to cystoscopy and TUR. Fluorescence imaging is switched on and off dynamically through the press of a button on the camera head. Fluorescence imaging is used for generalized survey of the urothelium as well as in conjunction with standard TUR, with improved contrast-enhanced visualization of both papillary and flat (i.e., carcinoma in situ, CIS) lesions.
In diagnostic cystoscopy, PDD is reported to have a sensitivity and specificity ranging from 87 to 97 % and 43 to 76 %, respectively [4]; a meta-analysis demonstrated that 5-ALA and HAL had similar sensitivity and specificity rates [6]. PDD has been shown to have an improved rate of initial detection of papillary and CIS lesions compared to WLC [7–9]. A meta-analysis of three phase III studies concluded that HAL had a higher CIS detection rate than WLC [10]. In addition, several meta-analyses have found significantly reduced residual tumor rates with PDD, with odds ratio of residual tumor being 0.28 (95 % CI, 0.15–0.52) compared to WLC [11] and the RR of residual tumor being 2.77-fold higher (95 % CI, 1.47–5.02; p = 0.002) with WLC versus PDD [12].
However, the overall recurrence rate of PDD-guided TUR of bladder tumor for NMIBC remains to be determined. One extensive meta-analysis compiled 1,345 patients across six studies with known or suspected NMIBC comparing HAL to WLC, with significant additional lesions detected (p < 0.001) and a significantly lower recurrence rate at 12 months in the HAL group versus WLC group (34.5 % vs 45.4 %; p = 0.006). When stratified by subgroup, recurrence rates were lower for patients with T1/CIS (p = 0.052) and Ta disease (p = 0.04) and high-risk (p = 0.05) and low-risk (p = 0.029) subgroups [13]. On the other hand, two studies found no significant difference in decreasing recurrence risk or progression-free survival with fluorescence cystoscopy [7, 9]. Further randomized trials with longer follow-up time periods will be needed to validate the long-term efficacy of PDD in determining a significant benefit in recurrence-free and progression-free survival.
Confocal Laser Endomicroscopy
CLE provides dynamic, high-resolution, subsurface imaging of the mucosa. While conventional confocal microscopes are bulky instruments widely used in laboratory settings [14–16], recent advances in instrument miniaturization have led to the development of flexible, fiberoptic endomicroscopes that can be passed through the working channel of standard endoscopes. Similar to confocal microscopy, in CLE, the illuminating light from a fiber is focused by an objective lens onto a tissue. Whereas scattered light from the in-focus tissue plane converges back into the fiber, the light from out-of-focus planes is inefficiently collected.
Of the different imaging technologies available clinically, CLE provides the highest spatial resolution (1–5 μM). Optical sectioning is performed using a 488-nm laser as the light source. Fluorescein, an FDA-approved drug, is used as the contrast agent. CLE has been approved for gastrointestinal and pulmonary endoscopic applications, with recent approval for the urinary tract.
The primary clinical system (Cellvizio, Mauna Kea Technologies) (Fig. 1.1b) uses imaging probes ranging from 0.85 to 2.6 mm in diameter that are passed through the working channels of standard endoscopes to examine the urinary tract. Images are acquired as video sequences at 12 frames/s, which allows real-time dynamic imaging of physiologic processes such as vascular flow. The images are reminiscent of standard histopathology with microarchitecture and cellular features.
In vivo applications of CLE in bladder surgery involve intravesical or intravenous administration of fluorescein with minimal systemic toxicity (Fig. 1.3) [17]. After an initial pilot study demonstrating the feasibility of using CLE in ex vivo bladders after cystectomy [18], CLE was conducted in 27 patients undergoing cystoscopy under anesthesia and TUR [19]. The ability to differentiate normal/benign mucosa from cancer (low- and high-grade lesions) was demonstrated (Fig. 1.4). Features of low-grade disease include densely packed cells and presence of fibrovascular stalks; on the other hand, high-grade disease exhibits a more distorted microarchitecture with pleomorphic, irregularly shaped shells with loss of cellular cohesiveness, indistinct cell borders, and disorganized vasculature. Carcinoma in situ (CIS) also contains a population of pleomorphic cells and indistinct cell borders but may also have extensive acellular areas that may be secondary to areas of denuded urothelium. In contrast, inflammatory sites (from processes such as post-BCG granulomatous reaction, Foley catheter reaction, urinary tract infection, scarring from prior resection) contain loose aggregations of small monomorphic cells consistent with local recruitment of leukocytes in the lamina propria. Similar to histopathology, the superficial umbrella cell layer is commonly absent in cancerous lesions.
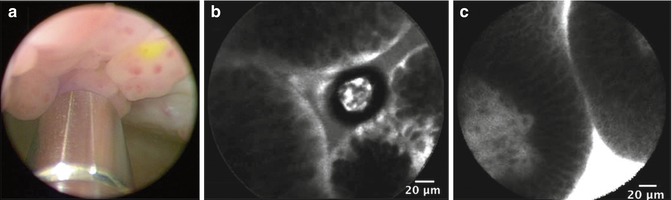
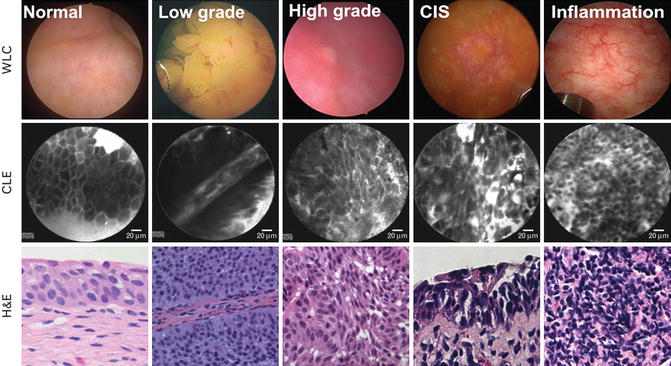

Fig. 1.3
Representative images from WLC and CLE with intravesical or intravenous administration of fluorescein. (a) WLC with intravesically stained papillary tumor. 2.6-mm imaging probe is visible at the bottom of the image. (b) CLE of intravesically stained papillary tumor. (c) CLE of intravenously stained papillary tumor (From Chang et al. [17], with permission)

Fig. 1.4
Probe-based optical biopsy with CLE of the bladder. CLE of normal, low/high-grade papillary bladder cancer, carcinoma in situ (CIS), and inflammation are shown with their corresponding white-light cystoscopy images and hematoxylin and eosin (H&E) staining from the subsequent biopsy site. Low-grade cancer shows characteristically organized papillary structures, in contrast to high-grade cancer and CIS which display pleomorphic cells and distorted microarchitecture. The inflammatory setting shows lymphocytic infiltrates (From Hsu et al. [27], with permission)
An imaging atlas was created to establish criteria for CLE diagnosis of bladder cancer [20]. Distinguishing anatomic features were documented among the various parts of the urinary tract, including the kidney (ex vivo), ureter (ex vivo), bladder, urethra, and prostate. In addition, the image process technique of mosaicing was used, allowing for juxtaposition of overlapping images into a composite, panoramic image. This application enables expansion of the field of view while preserving image fidelity.
More recently, an interobserver agreement study of CLE was conducted among novice and experienced CLE users. CLE was found to be a highly adoptable tool for cancer diagnosis and in novice CLE observers with moderate interobserver agreement. Experienced CLE observers, on the other hand, attained substantial levels of agreement for cancer diagnosis that was higher than for WLC alone [21].
Conclusion/Future Studies
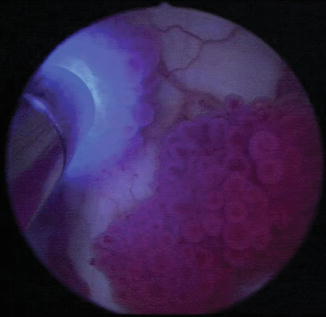
PDD and CLE hold promise in redefining the urologist’s approach to examining the lower urinary tract with cystoscopy. While both technologies have potential implications in improving diagnosis and treatment, they also have inherent limitations that one must be aware of and also have costs in terms of instrumentation, contrast agents, and additional operative time. For example, with PDD, false fluorescence can result if the light source is applied tangentially to the area of interest [12, 22], and an up to 30 % false-positive rate has been reported, particularly in patients with prior BCG treatment and during the practitioner’s learning curve [23]. On the other hand, CLE’s maximal depth of penetration is only about 120 μM. In the case of the bladder, it is insufficient to provide information about muscle invasiveness as it can only provide anatomic detail to the level of the lamina propria. In addition, CLE requires that the probe be in direct contact with the target tissue, thus precluding imaging of the entire urothelium in a single instance given its narrow field of view. To try to improve on this drawback, CLE may be combined with other imaging modalities to help with localization, including PDD (Fig. 1.5), but the combination of HAL and CLE precluded histologic analysis by CLE because of the excitation/emission spectra of HAL [24]. Other fluorochromes are being investigated for their potential in this strategy of readily identifying lesions with real-time histologic confirmation. Future studies will focus on the diagnostic accuracy of CLE for bladder cancer, particularly the challenging flat, erythematous lesions. In addition, CLE may be expanded to other urologic applications, including the upper urinary tract and during laparoscopic and robotic-assisted surgery.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








