Endoscopic Diagnosis, Staging, and Management of Gastrointestinal Cancers
Marcia Irene Canto
Endoscopic Diagnosis and Techniques
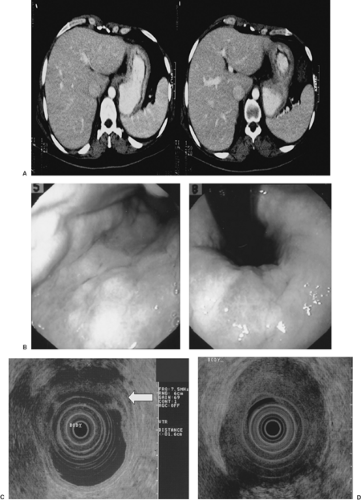
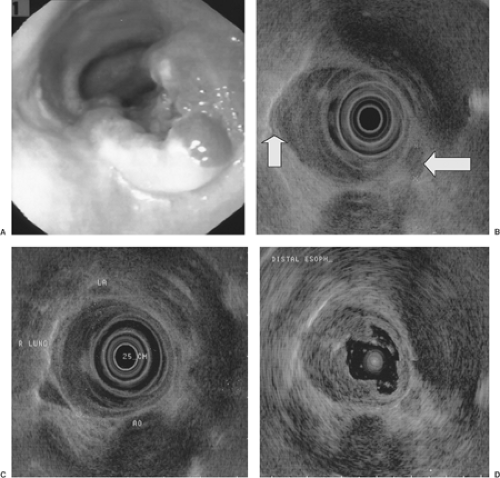
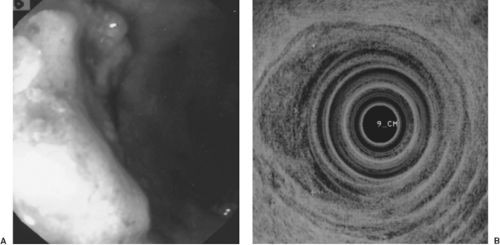
Gastrointestinal (GI) videoendoscopy has been a standard technique for visualizing and diagnosing GI cancers and their premalignant lesions. In addition, tissue samples in the form of brushings and mucosal biopsies are readily acquired for a pathological diagnosis (1). The most common clinical indications for performing videoendoscopy for suspected cancer are blood in the stool, chronic iron-deficiency anemia, change in bowel habits, anorexia, and dysphagia. Typically, GI cancers present as an ulcer, luminal narrowing (stricture), marked wall thickening (Fig. 10.1), and/or mass (Figs. 10.2 and 10.3).
In addition to standard videoendoscopy, endoscopic ultrasonography (EUS) was developed in the 1980s to improve visualization of the pancreas by allowing a transgastric or transduodenal placement of the ultrasound (US) probe unimpeded by bowel air. EUS combines videoendoscopy and high-frequency ultrasonography (Fig. 10.4). It allows visualization of the entire GI tract wall. Since the mid-1980s, it is still the best imaging modality of the GI tract wall. Importantly, it can also image adjacent organs such as pancreas, bile duct, adrenal gland, and liver (2). Hence, it has become an important diagnostic and staging modality for GI and pancreaticobiliary cancers. It is superior to dual-phase computed tomography (CT) for detection of pancreatic cancer (3,4) (particularly early cancers <2 cm [5,6]) and pancreatic neuroendocrine tumors (7). The negative predictive value of EUS for excluding a pancreatic tumor or mass in patients with a clinical suspicion of pancreatic cancer is very high (100%) (4), potentially obviating the need for further diagnostic testing when used as a first test (8).
In the 1990s, it became possible to obtain tissue samples from organs outside the GI tract via EUS guidance (EUS-guided fine-needle aspiration [EUS-FNA]) and a cytologic diagnosis of cancer in primary organs (pancreas, bile duct, gallbladder), lymph nodes, and distant organs, such as liver, adrenal gland, periportal area, and pancreas. The overall accuracy rate for EUS-FNA for diagnosing pancreatic cancer in suspicious lesions is 89% (compared to 74% for multidetector pancreatic protocol CT [4]) and 92% if there is no mass by CT (4). In another large prospective study, the sensitivity of EUS was 98% compared to 86% for multidetector CT detection of pancreatic cancer (9). EUS has a 22% negative predictive value in patients with obstructive jaundice, and 89% in those without obstructive jaundice (4). Hence, FNA does not seem to add much to the EUS alone in the diagnosis of pancreatic cancer in patients with obstructive jaundice. It can be argued that it is not necessary prior to planned surgery except in two scenarios: (i) when the patient is unsure about surgery or has multiple medical comorbidities that increase risk for surgical complications, and (ii) if the patient will be treated in a neoadjuvant protocol before surgery and a histologic diagnosis is required.
Endoscopic Screening and Surveillance
Patients at risk for the development of GI cancer can be identified by endoscopic screening and followed periodically using a variety of endoscopic techniques. Endoscopic screening with colonoscopy is commonly performed for average- and high-risk populations (13) (Tables 10.1 and 10.2). Both cancer and the premalignant adenomas can be accurately detected. Colonoscopy offers the advantages of complete visualization of the entire colon, detection and removal of polyps, and diagnostic sampling of cancers (14). Furthermore, colonoscopic polypectomy has been shown to significantly reduce the expected incidence of colorectal cancer by 76% to 90% in multiple cohort studies. In the National Polyp Study, patients who underwent colonoscopy with polypectomy had a 76% reduction in colorectal cancer incidence compared with a general population registry (15). Surveillance of patients with resected colonic adenomas for metachronous lesions is also recommended, depending on the type and number of adenomas detected at the index colonoscopy (16). People at increased risk have three or more adenomas, high-grade dysplasia, villous features, or an adenoma ≥1 cm in size. It is recommended that they have a 3-year follow-up colonoscopy. People at lower risk who have one or two small (<1 cm) tubular adenomas with no high-grade dysplasia can have a follow-up in 5 to 10 years, whereas people with hyperplastic polyps only should have a 10-year follow-up as average-risk people (16). Endoscopic screening of patients at risk for Barrett esophagus (BE)/esophageal adenocarcinoma and squamous dysplasia/carcinoma can also detect early neoplastic lesions (Table 10.1).
Screening of high-risk individuals with an inherited predisposition for familial esophageal gastric, colorectal, and pancreatic cancer is also performed (Table 10.2), with a view to potentially therapeutically intervene prior to the development of cancer. For example, endoscopic screening with chromoendoscopy performed on relatives from families with hereditary diffuse gastric cancer (E-cadherin mutation) is initiated at age 16 years after genetic testing (17,18). Screening with unsedated transnasal endoscopy can diagnose BE in asymptomatic
relatives from familial BE kindreds (19). Also, diagnosis of the precancerous lesion, such as the intraductal papillary mucinous neoplasm, can be made by EUS-FNA and surgical resection can be offered to asymptomatic relatives from familial pancreatic cancer kindreds (20) prior to the development invasive, incurable pancreatic adenocarcinoma.
relatives from familial BE kindreds (19). Also, diagnosis of the precancerous lesion, such as the intraductal papillary mucinous neoplasm, can be made by EUS-FNA and surgical resection can be offered to asymptomatic relatives from familial pancreatic cancer kindreds (20) prior to the development invasive, incurable pancreatic adenocarcinoma.
Endoscopic Staging of Luminal Cancers
The main endoscopic staging modality for GI cancers is EUS. It is superior to CT scan for staging the depth of invasion of esophageal (21,22) (Fig. 10.2), gastric (23) (Fig. 10.1), and rectal cancer (24) (Fig. 10.3). EUS is better than or comparable to CT and comparable to or better than positron emission tomography for regional lymph node metastasis from esophageal cancer (25). EUS is also better than standard pelvic magnetic resonance imaging (MRI), endorectal MRI (26), and CT for staging depth of tumor invasion, but equal to CT (24) and inferior to MRI for regional lymph node metastases for rectal cancer (27). Diagnosis of clinically suspected tumor regional esophageal (28), gastric (28), and rectal (26) cancer recurrence are better with EUS than CT or MRI. EUS is equal to multidetector, dual-phase pancreatic protocol CT for primary tumor assessment and locoregional staging of pancreatic cancer (3).
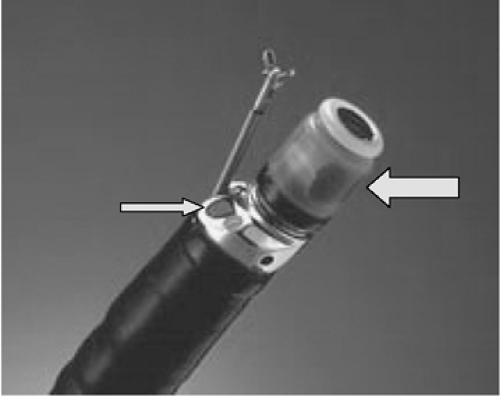 FIGURE 10.4. Radial scanning echoendoscope (Olympus America) used for endoscopic ultrasonography showing mechanical ultrasound transducer (left arrow) and oblique-viewing optics (right arrow). |
The accuracy of EUS for staging GI cancer is highest with advanced tumors that penetrate through the muscularis propria (T3) (Fig. 10.1) (accuracy rates usually ≥85%) and have regional lymph node involvement (N1) (accuracy rates typically 65%–75%). EUS staging results may impact patient management, particularly for esophageal (29) and rectal cancers (24) that are often treated with neoadjuvant chemoradiation therapy before surgery. EUS is generally least accurate with T2 lesions due to microscopic invasion into or through the muscularis propria that cannot be visualized. There has also been a need to improve the staging accuracy for T1 cancers because of the possibility of curative therapy of mucosal cancers with endoscopic mucosal resection (EMR) and/or photodynamic therapy. High-frequency (12-, 20-, and 30-Mhz) EUS probes (30) (Fig.10.2D) have been developed to improve the lateral resolution and help visualize the muscularis mucosae and submucosa. A few studies have shown improvement in staging accuracy of T1 mucosal and submucosal cancers in the esophagus (30) and stomach. Standard EUS with 5- and 7.5-Mhz frequencies (penetration depth of 6–10 cm) still needs to be performed with high-frequency EUS because the latter has limited depth of penetration (to 1–2 cm or less) and may miss regional lymph nodes. High-grade malignant esophageal strictures that do not allow passage of the echoendoscope can be staged with a 7 mm narrower instrument, the “blind” esophagoprobe (the standard echoendoscope minus the optics) that can be passed over a guidewire across the stricture (30) or a 2-mm catheter US probe (Fig. 10.2D).
When the accuracy rates for TNM (tumor, node, metastasis) staging for cancer at various sites are compared (Table 10.3), the highest reported accuracy rates are with esophageal and
rectal cancer and lowest for gastric cancer, presumably due to peritumor inflammation and absence of the serosal layer in certain parts of the stomach (31).
rectal cancer and lowest for gastric cancer, presumably due to peritumor inflammation and absence of the serosal layer in certain parts of the stomach (31).
Table 10.1 At-Risk Groups Eligible for Endoscopic Screening in the United States | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
EUS-FNA of regional and distant metastatic lymph nodes in the celiac, perigastric, and mediastinal regions increases the accuracy for N and M staging (32,33). Furthermore, detection and aspiration of small amounts of ascites not detected by CT or US can upstage cancer patients with occult peritoneal carcinomatosis (34).
Endoscopic Staging for Pancreatic and Biliary Cancer
EUS is highly accurate for staging primary pancreatic adenocarcinoma. Older studies had reported superior accuracy rates for T staging compared to CT (e.g., EUS 92% for T1, 85% for T2, and 93% for T3 lesions vs. 65%, 67%, and 38% for CT [35]), using surgical pathology as the reference standard. Furthermore, more recent studies using multidetector pancreatic protocol CT show variable results for the staging accuracy of EUS compared to CT, which may be due to interobserver variability for EUS and CT and technical differences in the CT equipment and protocol. A large single-center study reported that EUS is superior to CT for T staging (67% vs. 41%, p = 0.012) and equivalent for nodal staging (44% vs. 47%) (9). However, these accuracy rates are lower than reported by other studies for both EUS and CT. EUS is also equivalent to CT for prediction of pancreatic cancer resectability based on vascular involvement (88% vs. 92%) (Fig. 10.5) (9). EUS is superior to angiography for detecting vascular invasion (36).
Table 10.2 High-Risk Groups Eligible for Endoscopic Surveillance | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree