Fig. 7.1
Five-layer anatomic model of an ERUS scan. Three hyperechoic (white) layers and two hypoechoic (black) layers are visualized. A anterior, L left, P posterior, R right, T transducer
First hyperechoic layer: Interface between the balloon and the rectal mucosal surface
Second hypoechoic layer: Mucosa and muscularis mucosa
Third hyperechoic layer: Submucosa
Fourth hypoechoic layer: Muscularis propria
Fifth hyperechoic layer: Interface between the muscularis propria and perirectal fat
To aid in memory, a “Big Mac” analogy has been suggested with three white areas representing the buns and two dark areas representing the meat (muscularis mucosa or propria).
Occasionally, a seven-ring model may be visualized when the muscularis propria is observed as two black rings separated by a white ring (Fig. 7.2). This model represents the inner circular and outer longitudinal muscle layers as hyperechoic (black) rings separated by a hypoechoic (white) interface.
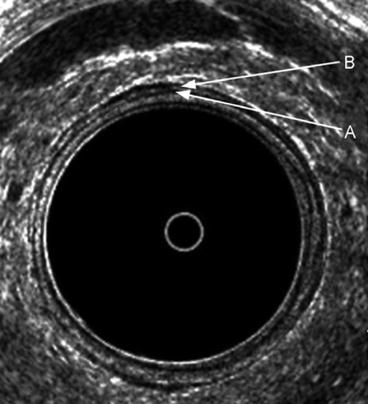

Fig. 7.2
The typical five layers of the rectal wall. Seven layers are depicted anteriorly, where an interface can be seen between the inner circular A and outer longitudinal B muscle layers of the muscularis propria
Assessment of Rectal Neoplasms
Depth of Invasion
Ultrasound staging classification (uTNM) is presented in Table 7.1.
Table 7.1
Ultrasound staging classification (uTNM) for rectal cancer
uT0
Noninvasive lesion confined to the mucosa
uT1
Tumor confined to the mucosa and submucosa
uT2
Tumor penetrates into but not through the muscularis propria
uT3
Tumor extends into the perirectal fat
uT4
Tumor involves an adjacent organ
uN0
No evidence of lymph node metastasis
uN1
Evidence of lymph node metastasis
uT0 Lesions
uT0 lesions are benign, noninvasive lesions confined to the rectal mucosa. Sonographically, the mucosal layer (inner black band) is expanded with an intact submucosa (middle white, hyperechoic line) (Fig. 7.3).
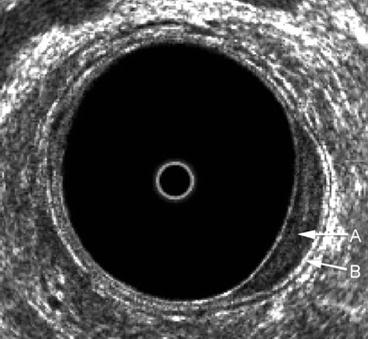
Fig. 7.3
A benign uT0 lesion in the left posterolateral aspect of the rectum. There is an expansion of the inner black line that represents the mucosa A, but the submucosa B is seen to be completely intact
Benign rectal villous adenomas are classified as uT0 lesions and may be treated with local excision with excellent results. Important in this decision is to accurately exclude any focus of invasion.
The accuracy of ERUS is probably highest for T0 lesions.
uT1 Lesions
uT1 lesions are early invasive cancers. uT1 lesions have invaded the mucosa and submucosa without penetrating into the muscularis propria. Sonographically this is characterized by an irregular middle white line (submucosa) without alteration of the outer black line (muscularis propria) (Fig. 7.4). Irregularities are indicated by a thickening or stippling of the submucosal layer, but there must not be a distinct break in the submucosal layer. A distinct break in the submucosal (middle white line) layer indicates invasion of the muscularis propria, hence a T2 lesion.
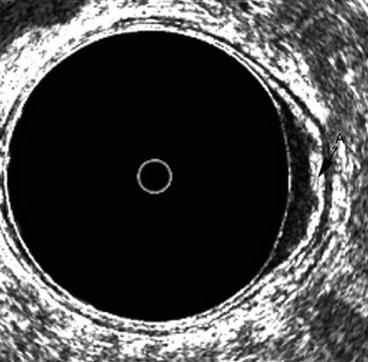
Fig. 7.4
A uT1 cancer in the left lateral wall of the rectum. The middle white line or submucosa is irregular and somewhat thickened A but not completely disrupted
uT2 Lesions
uT2 lesions penetrate into the muscularis propria (second hypoechoic, black line) but are confined to the rectal wall. Sonographically the hallmark finding is a distinct break in the submucosal layer. Characteristically, there is an expansion of the muscularis propria (outer black line), but the interface between the muscularis propria and the perirectal fat (the outermost white line) remains intact. The expansion of the muscularis propria may be variable depending on the degree of invasion.
“Early” uT2 lesions may just invade the muscularis propria with minimal expansion of the layer. “Deep” uT2 lesions have significant expansion of the muscularis propria (outer black line) and may appear to scallop the outer aspect of the muscularis propria but preserve the interface with the perirectal fat.
uT3 Lesions
uT3 lesions penetrate the full thickness of the muscularis propria and into the perirectal fat. Contiguous structures are not involved. The sonographic appearance reveals disruption of the submucosa, thickening of the muscularis propria, and disruption of the outer hyperechoic, white line indicating penetration into the perirectal fat (Fig. 7.5). The recognition of perirectal fat invasion is an important determinant in the preoperative evaluation of the rectal cancer patient.
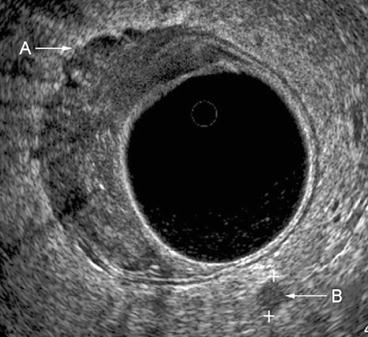
Fig. 7.5
A uT3N1 lesion. The tumor disrupts all layers of the rectal wall, with extensions evident into the perirectal fat A. A lymph node B is identified in the left posterior location within the mesorectum
uT4 Lesions
uT4 lesions are locally invasive into contiguous structures such as the uterus, vagina, cervix, bladder, prostate and seminal vesicles, or involve the pelvic sidewall or sacrum. They are clinically fixed and tethered. Sonographically, there is loss of the normal hyperechoic interface between the tumor and adjacent organ (Fig. 7.6).
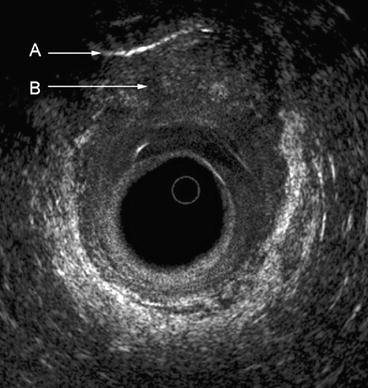
Fig. 7.6
A T4 lesion in the distal rectum and upper anal canal extending to the vagina. The curved white line A seen anteriorly represents the examiner’s finger in the vagina, and the hypoechoic anterior tumor B can be seen to extend into the vagina
Nodal Involvement
Unfortunately, the accuracy of detecting involved lymph nodes is less than the accuracy in determining the depth of invasion. The accuracy of ERUS in detecting lymph node metastases ranges from 50 to 83 %. ERUS determination of metastatic lymph nodes is certainly more accurate than clinical (digital) evaluation as well as other imaging modalities including computed tomography (CT).
As indicated in Table 7.1, lymph node staging parallels pathologic TNM staging classifying tumors with (uN1) or without (uN0) lymph node involvement. Undetectable or benign-appearing lymph nodes are classified as uN0. Malignant-appearing lymph nodes are classified as uN1.
Normal, nonenlarged lymph nodes are usually not detectable by ERUS. Inflamed, enlarged lymph nodes appear hyperechoic with irregular borders. Lymph nodes suspicious for malignancy include larger, round, hypoechoic lymph nodes with an irregular contour.
Hypoechoic lymph nodes greater than 5 mm are highly suspicious for metastases. Involved lymph nodes are usually found adjacent to the primary tumor or within the proximal mesorectum.
ERUS differentiates two main groups of lymph nodes: hypoechoic and hyperechoic lymph nodes.
Compared with pathologic findings, hypoechoic lymph nodes represent metastases, whereas hyperechoic lymph nodes are visualized because of nonspecific inflammation.
There is no definitive size threshold to determine if an identified lymph node is malignant.
Overall, four nodal patterns are seen with differing probabilities of being involved with metastatic disease. Nonvisible lymph nodes on ultrasound have a low probability of harboring lymph node metastases.
Hyperechoic lymph nodes with nonsharply delineated boundaries are more often benign resulting from inflammatory changes. Hypoechoic lymph nodes larger than 5 mm are highly suggestive of lymph node metastases.
Mixed echogenic lymph nodes larger than 5 mm are difficult to classify but should be considered malignant.
Accurate lymph node staging of rectal cancers by ERUS relies on the experience of the examiner. False-positive results may occur because of inflammatory lymph nodes or confusing the cross-sectional appearance of perirectal blood vessels for metastatic lymph nodes.
Scanning longitudinally will distinguish between blood vessels and lymph nodes because blood vessels will extend longitudinally, change direction, and/or branch. The sonographic continuity of the hypoechoic vessel over a distance greater than the cross-sectional area is the criterion used to distinguish the two. Three-dimensional imaging can help in making this distinction.
False-negative results are also problematic in interpreting nodal involvement on ERUS. Lymph nodes harboring micrometastases are difficult to detect. Grossly malignant lymph nodes may be present outside the range of the ultrasound probe and remain undetectable. This may be the case of lateral pelvic lymph nodes such as the obturator nodes as well as those within the mesorectum beyond the proximal extent of the rigid probe.
Accuracy of Ultrasound in the Diagnosis of Rectal Cancer
The accuracy of ERUS for tumor depth of invasion has been reported in the range of 63–93 % (Table 7.2).
Table 7.2
Accuracy of ERUS in the staging of rectal cancer
Author
Year
n
Accuracy (%) T stage
Accuracy (%) N stage
Hildebrandt and Feifel
25
92
n/a
Saitoh et al.
88
90
75
Holdsworth et al.
36
86
61
Beynon et al.
100
93
83
Rifkin et al.
102
65
50
Glaser et al.
86
88
79
Jochem et al.
50
80
73
Milsom and Graffner
52
83
83
Orrom et al.
77
75
82
Katsura et al.
112
92
n/a
Glaser et al.
154
92
81
Herzog et al.
118
89
80
Deen et al.
209
82
77
Akasu et al.
152
82
76
Adams et al.
70
74
83
Kim et al.
89
81
64
Garcia-Aguilar et al.
545
69
64
Marusch et al.
422
63
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access




