Fig. 12.1
ELUS probe and system. (a) Photograph of a 5.4 French (1.8 mm) ELUS probe. This probe can be passed over a guidewire, and it has a shielded transducer, which continuously rotates to provide radial images with adjustable scanning depths of 1–6 cm. (b) Photograph of the ELUS system setup at our institution. The actual system generator is hidden underneath the keyboard; a cable that houses the cable drive and electronics connects the generator to the probe, which is carefully placed in a holder and kept sterile with surrounding plastic drape. The monitor, recording capability, and cart are not part of the standard system but were put together by operating room personnel. (c) Photograph of system keyboard (All images copyright S.F. Matin 2013)
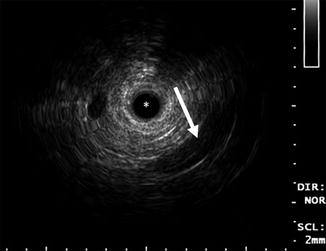
Fig. 12.2
Typical ELUS images of the left mid-distal ureter. The catheter (asterisk) occupies the distal ureter, which is not distended. The iliac vessels are seen posterior and lateral to the ureter in the 5 o’clock position (arrow). An ultrasonographic shadow is generated by the guidewire (tail of arrow) (Copyright S.F. Matin 2013)
For ELUS imaging of the ureter and renal pelvis, retrograde pyelography is initially performed to evaluate the tissue for multifocality and to confirm the location of the index tumor. A guidewire is passed through the ureter, and the ELUS probe is placed over this and under fluoroscopy is passed to the site of the index tumor. The ultrasonographic image gain and contrast are adjusted to produce an anechoic signal for the surrounding fluid in the ureteral and renal pelvic lumen for optimal image acquisition. The imaging depth is then adjusted to obtain optimal viewing and resolution (usually at 2–3 cm). The probe is passed methodically from below to above the tumor, and any abnormalities suggestive of invasion are noted (discussed below). A combination of intermittent fluoroscopy to view the position of the probe and recognition of anatomic landmarks is necessary for real-time interpretation of endoscopic ultrasonographic images. Once adequate imaging and documentation are completed, the probe is withdrawn, and ureteroscopic evaluation with biopsy and any other adjunctive procedures, such as stent placement, is performed.
Interpretation of ELUS Images
While normal urologic anatomy is easily recognizable with ELUS, some familiarity with axial ultrasound images is helpful. The ureters traverse anterior to the iliac vessels at the level of the lumbar-sacral joint (Fig. 12.2). The segment inferior to the iliac vessels is the distal or pelvic ureter, and the segment superior to the iliac vessels to the pelvi-ureteric junction is the abdominal ureter. The capacious renal pelvis is usually filled with fluid and lacks the cylindrical contour of the ureters. The renal vein, which is anterior to the pelvis, is a useful landmark for identifying the anterior and posterior pelvic walls (Fig. 12.3).
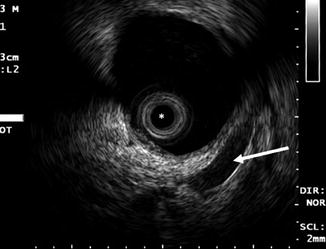

Fig. 12.3
Typical ELUS image of the right renal pelvis. The renal pelvis is distended and filled with fluid. The mucosal layer is poorly visible; the muscularis layer itself is hypoechoic compared with the surrounding parenchyma and sinus fat. Reverberation artifact can be seen around the transducer (asterisk). The renal vein (arrow) is anterior to the pelvis and can be used to identify the anterior and posterior walls of the pelvis (Copyright S.F. Matin 2013)
Ureteral vessels, which are branches of renal, internal gonadal, hypogastric, or inferior vesicles, are seen as hypoechoic tubular structures running parallel to the ureteral lumen (Fig. 12.4). Three distinct layers of the ureters can be appreciated on high-frequency ultrasonography: an inner hyperechoic mucosa (the transitional epithelium), a middle hypoechoic layer comprised of circular and longitudinal muscles, and an outer hyperechoic layer which merges with the hyperechoic periureteric fat (which is composed of loose fibrous tissue) (Fig. 12.5). Renal parenchyma and pyramids are visible adjacent to the outer layer in the calyces.
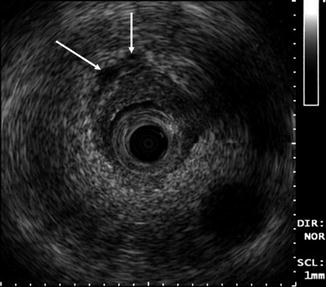
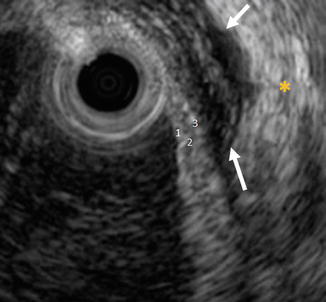

Fig. 12.4
Typical ELUS image of the distal ureter. Ureteral vessels (arrows) run parallel to the ureter. These can be identified on real-time imaging and appear as hypoechoic tubular structures compared with periureteric fat (Copyright S.F. Matin 2013)

Fig. 12.5
Three subtle but distinguishable layers of the ureter appear as alternating hyperechoic and hypoechoic bands. The inner hyperechoic mucosa (1), the middle hypoechoic muscularis (2), and the outer hyperechoic adventitious layer (3). The outer layer is difficult to appreciate in most examinations because it merges with the hyperechoic periureteric fat (yellow asterisk) unless separated by periureteric fluid (arrows) (Copyright S.F. Matin 2013)
Inflammatory and neoplastic lesions are difficult to differentiate on ELUS. Most lesions are isoechoic to the intermediate muscle layer or exhibit slightly low echogenicity. In a non-distended ureter, these lesions are seen as eccentric masses surrounding the ultrasound probe (Fig. 12.6). Visualization of the three sonographically distinct layers in the ureter should allow accurate assessment of the depth of invasion of tumor, similar to the experience of ELUS with assessing various gastrointestinal malignancies [6, 7]. Invasive tumors can be seen extending into the middle hypoechoic layer comprising the muscularis. Extension into the periureteric fat can be challenging to diagnose because the outer adventitious layer often has an echogenicity similar to that of the surrounding periureteric fat [8]. Lobularity (distortion of the smooth round or ovoid contours) of the outer surface of the ureter indicates periureteric spread of a lesion (Fig. 12.7).
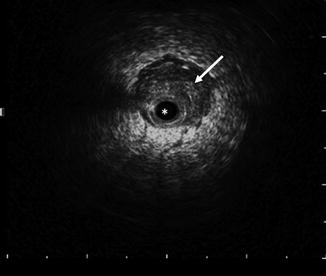
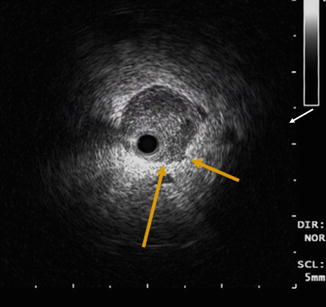

Fig. 12.6
ELUS image of the right ureter of an UTUC. The catheter (asterisk) occupies the non-distended ureter, which shows eccentric thickening (arrow) compatible with urothelial tumor. The wire shadow is visible at the 9–10 o’clock position (Copyright S.F. Matin 2013)

Fig. 12.7
ELUS image of a mass in the right ureter of an UTUC at the level of the ureteropelvic junction. Loss of the smooth contour of the outer surface of the ureter (arrows) suggests periureteric spread. Periureteric vessels are indicated by the white arrow (Copyright S.F. Matin 2013)
If the ureter is not distended, the differentiation of sessile and polypoidal lesions can be challenging. The concomitant use of ureteroscopy makes this limitation less critical. In a well-distended system and in the renal pelvis, it is often possible to make this differentiation without ureteroscopy (Fig. 12.8). Pedunculated tumors can be diagnosed from sonographic imaging results in a distended system when a stalk and its attachment can be seen. The presence of a fluid interface between the mass and the ureteric wall suggests a pedunculated mass (Fig. 12.9).
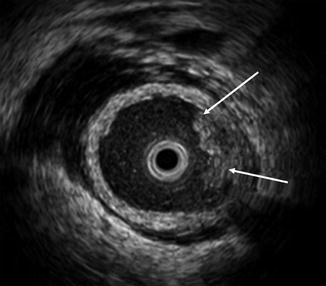
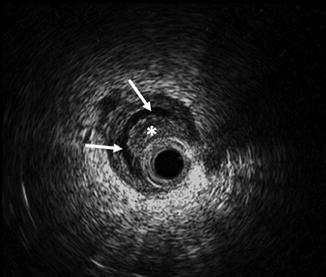

Fig. 12.8
ELUS image of a mass in the left ureter of a 70-year-old male with UTUC. The ureter is distended and exhibits minimal periureteric fluid. There is a sessile mass between the 1 and 4 o’clock positions (arrows) (Copyright S.F. Matin 2013)

Fig. 12.9
ELUS image of a mass in the left ureter of a 73-year-old female with UTUC. The mass (asterisk) is applied against the ultrasound catheter. A column of fluid (arrows) separates the mass from the ureteric wall (Copyright S.F. Matin 2013)
Use of ELUS in Urologic Surgery
Urethra
Very few studies have evaluated the use of ELUS in imaging the urethra. Chancellor et al. [9] assessed the usefulness of intraurethral sonography in a small case series of patients with urethral diverticula. Because a physical examination and clinical history are usually sufficient for a preoperative diagnosis, the role of ELUS in making this diagnosis is unclear. However, intraoperative ELUS improved the ability to perform complete dissection, ensured complete resection, helped to prevent injury, and helped in characterizing the diverticula [9].
Another potential application of ELUS is in the evaluation of the urethral rhabdosphincter [10, 11]. The capability of dynamic imaging using ELUS is advantageous and allows for real-time visualization. Klauser et al. [11] demonstrated that ELUS can distinguish morphologic changes in the sphincter mechanism; these changes translated to functional changes as measured by degree of urinary incontinence. In addition, ELUS was used by Rivas et al. [12] to evaluate and study the placement of periurethral collagen in a porcine system. The ability to use ELUS in relatively noninvasive fashion to evaluate the depth of collagen injection and to perform serial evaluations to assess durability of the collagen led the authors to conclude that submucosal injection was in optimal place. This animal model demonstrated the usefulness of ELUS for research purposes and scientific observation [12].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








