Chapter 10 ELECTROPHYSIOLOGIC EVALUATION OF THE PELVIC FLOOR
Clinical neurophysiologic tests have been proposed for research applications in patients with sacral dysfunction, but the emphasis in this chapter is on describing investigations with established diagnostic value. The roles of electrodiagnostic tests in various clinical conditions are described first, and brief descriptions of these investigative procedures are given at the end of the chapter.
CLINICAL APPLICATION OF SACRAL ELECTRODIAGNOSTIC TESTS
Electrodiagnostic tests are an extension of the clinical neurologic examination, and they are helpful in evaluating patients in whom a neurologic lesion is suspected. An international consensus statement proposes that sacral electrodiagnostic studies are most useful in patients with focal peripheral sacral lesions (i.e., conus medullaris, cauda equina, sacral plexus, and pudendal nerve lesions), in patients with multiple system atrophy, and in women with urinary retention.1 They can document the severity of a clinically diagnosed lesion and provide data on the integrity of various neurologic structures. However, these tests have limitations. They require trained personnel to be properly performed, they are not useful for screening, and they are uncomfortable for the patient. The results do not correlate well with clinical bladder, anorectal, or sexual dysfunction.
Neurogenic sacral organ dysfunction can be caused by a variety of neurologic disorders, but the value of sacral electrodiagnostic studies in such patients may be minor. In patients with brain and spinal cord disease, who may have pronounced pelvic organ complaints, imaging studies—magnetic resonance imaging (MRI) in particular—are more useful for establishing the underlying neurologic diagnosis. In this context, neurophysiologic testing outside the pelvic region may provide information about relevant abnormal spinal conduction, and somatosensory evoked potentials (SEPs) elicited by stimulation of the tibial nerve are more useful in these circumstances than pudendal SEPs.2,3 Similarly, in patients with sacral dysfunction due to a generalized peripheral neuropathy such as diabetes, nerve conduction studies in the lower limbs are a more sensitive adjunct to clinical examination than are sacral electrodiagnostic studies.4
ASSESSMENT OF PATIENTS BEFORE ELECTRODIAGNOSTIC TESTING
Clinical and laboratory evaluation of a woman with pelvic organ dysfunction is necessary before electrodiagnostic investigations can be considered. This order is followed so that there can be proper formulation of the questions for those in the clinical neurophysiology laboratory carrying out the tests. Examples of such questions are listed in Box 10-1.
ELECTRODIAGNOSTIC TESTING IN WOMEN WITH SACRAL COMPLAINTS
Incontinence after Childbirth
Research studies have used needle electromyography to examine the extent of nerve damage contributing to urinary stress incontinence after childbirth. The first studies using single-fiber electromyography (SFEMG) to look at fiber density showed partial reinnervation changes in the external anal sphincter (EAS)5 and pubococcygeus muscles in women with stress incontinence and genital prolapse.6 Needle electromyographic examination of the pubococcygeus muscle revealed a significant increase in motor unit potential (MUP) duration (i.e., an indication of reinnervation) after vaginal delivery, which was most marked in women with urinary incontinence 8 weeks after delivery, a prolonged second stage, and heavier babies.7 However, an electromyographic study, using less biased methods of automated MUPs and interference pattern analysis, questioned the widely held notion that significant damage to the innervation of the EAS occurs even during uncomplicated deliveries. Although vaginal delivery was related to minor electromyographic abnormalities, there was no indication that this correlated with loss of sphincter function.8 A histomorphologic study supported these data by failing to demonstrate significant neuropathic changes in pelvic floor muscles.9 In the urethral sphincter, in contrast, electromyography and muscle biopsy showed more neuropathic changes in women with stress incontinence than in controls.10 Even uncomplicated delivery may cause some distal pudendal nerve damage. Significant neurogenic damage proximal to the EAS muscle innervation probably occurs only rarely,11 and it is mainly caused by compression of the sacral plexus by fetal head.12 The prevalence and relevance of minor proximal injuries is unknown.
Kinesiologic electromyography performed using hook electrodes so that a prolonged recording could be made without causing discomfort showed some loss of coordination between the two sides of the pubococcygeus muscle in women with stress incontinence, implying an abnormal primary role of the central nervous system or a neurologic response to muscle or tendon damage.13 In addition to age-related neurogenic changes, the interference pattern changes consistent with motor unit loss, and failure of central activation has been found in the levator ani and EAS muscles of women with stress incontinence.14
Although extensively used in the past,15,16 the pudendal nerve terminal motor latency test is probably of no clinical use in women with urinary stress incontinence.11 Nerve latencies evaluate only the fastest nerve fibers and are therefore not sensitive to axonal loss, which is the major type of damage causing muscle denervation.
Detrusor Overactivity
Detrusor overactivity may result from neurologic disease or occur in an otherwise healthy individual, in which case the condition is called idiopathic detrusor overactivity. Clinical neurologic examination is the most useful means of differentiating these two entities. In addition to the clinical examination, imaging studies and electrodiagnostic tests of central nervous system conduction (i.e., motor evoked potentials [MEPs] and SEPs) may reveal underlying spinal cord disease, such as multiple sclerosis. In this respect, tibial SEPs are the most useful investigation.2,3
Extensive neurophysiologic investigations (e.g., electromyography of the EAS, bulbocavernosus reflex after dorsal clitoral nerve stimulation, tibial and pudendal SEPs, MEPs on cortical magnetic stimulation, recording from the EAS and abductor hallucis brevis muscles) in women with idiopathic detrusor overactivity failed to reveal any abnormality.17 No significant differences were reported in comparing this group with a group of 13 age-matched, healthy control women, thereby excluding even an occult neurologic abnormality. This result supports the view that idiopathic detrusor overactivity is caused by intrinsic bladder defects (i.e., neurogenic or myogenic). The role of electrodiagnostic investigations in detrusor overactivity is limited to establishing or excluding an underlying neurologic disease.
Urinary Retention
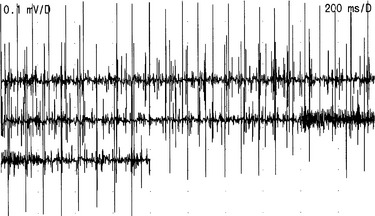
Isolated urinary retention in young women was formerly considered to be psychogenic or the first symptom of multiple sclerosis. However, needle electromyography of the urethral sphincter muscle has demonstrated that many such patients have profuse, complex, repetitive discharges and decelerating burst activity (Fig. 10-1).18 The cause of this activity is unknown, but an association with polycystic ovaries was described in a syndrome by Fowler and colleagues.19 The explanation probably lies with some unidentified hormonal susceptibility of the female striated urethral sphincter muscle that causes a loss of stability of the muscle membrane and permits direct muscle fiber to muscle fiber (ephaptic) transmission to develop, which manifests as complex, repetitive discharges. The current hypothesis is that the sustained contraction of the urethral sphincter has an inhibitory effect on the detrusor, resulting in urinary retention.
When recording from the striated urethral sphincter in this condition, only complex repetitive discharges (which sound like helicopters) may be heard, and the distinction between these and reinnervated motor units can be problematic, but if decelerating bursts (which sound like underwater recordings of whales) are also present, it is easier to be certain that the characteristic electromyographic activity has been recorded. Although electromyography may indicate the presence of an abnormality, it is inevitably only a very limited sample of the muscle activity in the immediate vicinity, and it is difficult to know whether the abnormality is sufficient to account for the clinical finding of complete or partial urinary retention. The investigations that have proved to be useful adjuncts are measurement of the urethral pressure profile and volume of the urethral sphincter muscle estimated with ultrasound.20 Young women with urinary retention due to the urethral sphincter abnormality often have urethral pressure profiles in excess of 100 cm H2O.
The typical clinical presentation of Fowler’s syndrome is of a young woman with spontaneous onset of urinary retention or retention after some sort of operative intervention. The mean age of a series of women with this problem was 27 years, and a spontaneous onset appears to be more common in women younger than 30 years.21 The woman may present with a bladder capacity in excess of 1 L, and although this may cause painful distention, she lacks any of the expected sensations of urinary urgency. There may or may not be a history of infrequent voiding before the onset of urinary retention. These women are taught to do clean, intermittent self-catheterization and commonly experience difficulties with the technique, particularly pain and difficulty in removing the catheter. A retrospective study of 248 women presenting with urinary retention over a 5-year period showed that this was by far the most common cause for urinary retention in young women.22
Patients with Fowler’s syndrome seem to respond particularly well to sacral neuromodulation.23 Although the mechanism of its action is still the subject of research using functional brain imaging methods,24 stimulation does not appear to lower the urethral pressure profile or cause a cessation of the abnormal electromyographic activity.25
The same type of abnormal spontaneous electromyographic activity may also occur in women with obstructed voiding. The electromyographic abnormality may persist during attempts at micturition,26 leading to interrupted flow, high detrusor pressure, low flow, and incomplete bladder emptying. It is thought that in this condition, the overactive urethral sphincter, although it produces obstruction, does not have the same inhibitory effect on the detrusor muscle as it does in women who become unable to void and develop urinary retention. Because needle electromyography of the urethral sphincter detects changes related to denervation and reinnervation, as well as this peculiar abnormal, spontaneous activity, it has been proposed that needle electromyography of the urethral sphincter muscle should always be undertaken in women with unexplained urinary retention.1,18
Anal Incontinence
Needle electromyography of the EAS was thought to be useful in patients with anal incontinence.27 However, there is no consensus regarding the utility of electrophysiologic testing in neurologically normal patients with isolated anal incontinence. In a series evaluated by Podnar and coworkers,28 patients with isolated anal incontinence rarely had neuropathic electromyographic changes in sacral segments. In a subgroup of patients in whom no cause of anal incontinence could be established (i.e., idiopathic anal incontinence), the only electrophysiologic abnormality found was a diminished number (absence) of continuously firing, low-threshold motor units during relaxation.28 In patients with fecal incontinence and an increased fiber density on SFEMG, lower anal squeeze pressures and diminished rectal sensation have been demonstrated. If marked changes of denervation and reinnervation are found in the EAS in the appropriate clinical setting, a more generalized disorder, such as multiple system atrophy or a cauda equina or conus medullaris lesion, should be considered. If performed, it is probably better that needle electromyography follows an anal ultrasound examination that excludes structural lesions of the sphincter mechanism.29
Chronic Constipation
Constipation occurs for a variety of reasons. Its prevalence depends on the diagnostic criteria applied. Radiographic methods can demonstrate prolonged colonic transit (using radiopaque markers) and abnormal pelvic floor movement during defecation (using cine defecography), which are the main mechanisms.30 Electromyography can be used to demonstrate continuous puborectalis muscle contraction characteristic of a subtype of obstructed defecation (i.e., nonrelaxing puborectalis syndrome),31 but this would be considered only if other investigations suggested that particular pathophysiology.
Chronic constipation with repetitive straining was thought to be the main cause of advancing pudendal neuropathy and increased fiber density identified on SFEMG in patients with urinary and anal incontinence.32 Semiquantitative or quantitative MUP changes on conventional electromyography of the EAS muscles of severely constipated subjects have been reported by some investigators. In a study using advanced MUP and interference pattern analysis, no abnormalities were demonstrated in the EAS muscles of patients with mild chronic constipation. This finding is important for the interpretation of electromyographic findings in patients with other conditions, a significant proportion of whom also suffer from chronic constipation.33
Sexual Dysfunction
Neurophysiologic techniques have been applied extensively in the research of male erectile dysfunction, but much less research has gone into female sexual dysfunction. Pudendal SEP recordings have been employed in women with sexual dysfunction due to spinal cord lesions, multiple sclerosis, and diabetes,34 but in a placebo-controlled trial of the effect of sildenafil citrate in women with sexual dysfunction and multiple sclerosis, the pudendal SEP was not found to be contributory.35 Pudendal SEPs usually have been found to be of no greater value than clinical examination in detecting relevant spinal cord disease.3