Chapter 48 Ectopic Pregnancy
INTRODUCTION
If undiagnosed or untreated, ectopic pregnancy may result in rupture of the fallopian tube and massive intraperitoneal hemorrhage. In the past decade, this phenomenon accounted for approximately 9% of maternal pregnancy-related deaths in the United States.1 Because ectopic pregnancy diagnosis and treatment has moved from an inpatient to an outpatient setting, there are no clear reporting standards, and the most up-to-date incidence rates and morbidity and mortality statistics date back to the mid-1990s.1
EPIDEMIOLOGY
It is estimated that about 2% of pregnancies in the United States are ectopic pregnancies. The number of ectopic pregnancies diagnosed in the United States continues to rise, with a sixfold increase documented between 1970 and 1992.2–4 In Europe, the prevalence of ectopic pregnancy appears stable in France, Sweden, and the Netherlands, but has continued to increase in Norway.4 U.S. healthcare statistics demonstrate that of the 108,800 patients diagnosed with ectopic pregnancy in 1992, 58,000 needed hospitalization, at a cost of $1.1 billion.5 Recent trends of outpatient treatment and decreased need for hospitalization have likely led to an underreporting of the condition and a consequent underestimation in U.S. government figures.
The vast majority of ectopic pregnancies (more than 90%) occur in the tube, with 80% to 90% of these occurring in the ampullary region, 5% to 10% in the isthmic region, 5% in the fimbrial region, and about 2.4% located in the cornual (interstitial) region. The other sites include 3.2% in the ovary, 1.3% elsewhere in the abdomen, and less than 0.15% in the cervix.6–8
Etiology
Ectopic pregnancies are believed to occur primarily as a result of factors that delay or prevent the passage of the fertilized egg into the uterine cavity. Partial or complete blockage of a fallopian tube may arrest the passing embryo in the fallopian tube lumen. Even if the tube is still patent, inflammation and infection may damage the endosalpinx of the tube. Resultant abnormalities in ciliary function are believed to impede transport of the embryo through the fallopian tube.9 The most common cause of such tubal damage is believed to be clinical or subclinical infections caused by Chlamydia trachomatis or Neisseria gonorrhoeae.
It is hypothesized that in some cases embryo factors (including cytogenetic abnormalities) may lead to premature implantation in a nonendometrial site. However, recent studies that have examined the role of chromosome abnormalities in ectopic pregnancy have not supported earlier case reports of high proportions of genetically abnormal gestations at ectopic sites. Among 22 surgically excised tubal pregnancies, only one abnormal chromosomal complement was found.10 In a larger review of 62 karyotyped ectopic pregnancies, 4.8% showed chromosomal abnormalities, a figure which matched that expected for maternal and gestational age of the cases.11
Risk Factors
Multiple risk factors have been consistently shown to be associated with ectopic pregnancies (Table 48-1).12 The strongest association has been found with prior pelvic inflammatory disease (PID), a prior history of ectopic pregnancy, and previous tubal surgery (including previous tubal ligation). These conditions are believed to alter tubal integrity and thus impede the migration of the fertilized ovum to the uterus.
Table 48-1 Risk Factors for Ectopic Pregnancy
Weaker associations have been made between ectopic pregnancy and infertility (a possible marker in some patients for subclinical tubal infection), cigarette smoking (thought to affect tubal motility), increasing age, having more than one lifetime sexual partner, any pelvic or abdominal surgery (other than cesarean delivery), and a history of having a sexually transmitted disease. Although use of an intrauterine device (IUD) does not increase the risk of ectopic pregnancy compared to controls, women using an IUD found to have a positive pregnancy test are more likely to have an ectopic than an intrauterine pregnancy, in a manner similar to women who have had a tubal ligation.13 Unfortunately, the sensitivity of these risk factors is low, and as many as 50% of patients with proven ectopic pregnancies will have none.13
No clear association has been documented between ectopic pregnancy and oral contraceptive use, previous elective pregnancy termination or spontaneous miscarriage, or cesarean section.9,10
Improved Diagnosis
Diagnostic accuracy has improved as a result of more sensitive pregnancy tests and pelvic ultrasound. The advent of radioimmunoassay and specific antiserum to the beta-subunit of human chorionic gonadotropin (hCG) has allowed for the accurate quantification of β-hCG and the ability to closely follow its rise and fall.14 High-resolution transvaginal ultrasonography with Doppler flow imaging has led to improved visualization of adnexal masses, including ectopic pregnancies, at earlier gestational ages.
Assisted Reproductive Technologies
Assisted reproductive technologies (ART) appear to increase the risk of ectopic pregnancies as a result of both preexisting pathology and effects inherent to these techniques. Not only do many subfertile women have tubal abnormalities, but ovulation induction also results in hormonal fluctuations that are hypothesized to alter tubal motility in some women. After oocyte retrieval, in vitro fertilization (IVF), and embryo transfer, the incidence of tubal pregnancy can be as high as 4.5%.15
Heterotopic Pregnancy
IVF also appears to increase the incidence of simultaneous intrauterine and ectopic pregnancy, termed heterotopic pregnancy.16 In the late 1940s, the prevalence of heterotopic pregnancy was estimated to be 1:30,000 pregnancies.17 The prevalence is now estimated to be 1:4000 pregnancies in the general population, and as high as 1:100 pregnancies resulting from IVF.15,18,19 This dramatic increase is believed to be the result of the increased risk of multiple pregnancies and the unknown effects on tubal motility, in combination with the invasive nature of ART. The clinician must be aware that although ultrasound verification of intrauterine pregnancy dramatically decreases the chance of an ectopic pregnancy, it does not completely rule it out, especially in patients whose pregnancy has resulted from ART.
Ruptured Ectopic Pregnancy
Fallopian tube rupture secondary to ectopic pregnancy remains relatively common despite greater awareness of the disease and improved diagnostic modalities. This is because there is little or no correlation between tubal rupture time since last menstrual period, physical findings, symptoms or β-hCG level. In a large retrospective study of 700 women with ectopic pregnancies, the rupture rate was 34% and one third of patients had no symptoms prior to rupture.20 The primary risk factors for tubal rupture in this study were no prior history of ectopic pregnancy and multiparity.
Likewise, there is little relationship between the onset of ectopic pregnancy symptoms and subsequent tubal rupture. In one study with an overall rupture rate of 32%, less than one fourth of the ruptures occurred within the first 48 hours of symptom onset.21 The remaining ruptures occurred at a fairly steady rate of 2.5% per 24 hours of untreated symptoms for the next 2 weeks.
It is surprising that there is no correlation between tubal rupture and β-hCG levels. In one study, 11% of patients with ruptured ectopic pregnancies had β-hCG levels less than 100IU/L.16 Even declining β-hCG levels are not clinically reassuring, because fallopian tube rupture can occur when serial β-hCG measurements demonstrate a dropping level, as well as when the β-hCG level is very low.22,23
PRESENTATION
Symptoms
Vaginal Bleeding
Vaginal bleeding occurs in more than 50% of ectopic pregnancies and can range from scant spotting to heavy bleeding. It is hypothesized that vaginal bleeding occurs because the thickened endometrial lining is not well supported by the abnormal hormonal milieu. Theoretically, lower β-hCG production associated with most ectopic pregnancies stimulates the corpus luteum to produce an inadequate amount of progesterone. Some patients may even report passing tissue vaginally. In such cases, it is important to keep in mind that a “decidual cast” from the endometrial cavity can easily be mistaken for tissue, and only microscopically confirmed chorionic villi should be used to confirm that the pregnancy was intrauterine.17
Physical Examination
Pelvic Examination
On pelvic examination, inspection of the cervix will usually reveal a closed os, with or without bleeding. A vigorous bimanual examination in search of an adnexal mass is contraindicated if an ectopic pregnancy is suspected. In the presence of adnexal tenderness, the size and characterization of any adnexal masses is best determined by ultrasound. Bimanual pressure on a fragile ectopic pregnancy can result in rupture, converting a stable patient into a surgical emergency. Even when an ectopic pregnancy is present, an adnexal mass will be palpable in more than 10% of cases, and in one third of these cases, the mass will ultimately prove to be unrelated to the ectopic pregancy (e.g., a corpus luteum cyst).17
DIAGNOSIS
Ultrasonography
Intrauterine Pregnancy Confirmation
The confirmation of an intrauterine pregnancy requires the identification of a series of structures by vaginal ultrasound, including the gestational sac, yolk sac, and fetal pole with or without cardiac motion. The gestational sac is seen first and appears as a thick, echogenic rim surrounding a sonolucent center in an eccentric location of the endometrial stripe. This is often referred to as the double decidual sign. The yolk sac is a bright echogenic rim with a sonolucent center that can be seen by approximately 5 weeks’ gestational age. The fetal pole develops as a thickening along an edge of the yolk sac, with cardiac motion first seen around 5½ to 6 weeks after the last menstrual period, even in the case of multiple gestations. The diagnostic accuracy of transvaginal ultrasound for identifying an intrauterine pregnancy approaches 100% in gestations greater than 5½ weeks.24
Adnexal Findings
Ectopic pregnancies can often be seen in the adnexa with vaginal ultrasound. The most common finding is an inhomogeneous mass, which has been reported to be visible in approximately half of patients with ectopic pregnancies in some series.25 Less commonly, a mass with a hyperechoic ring around the gestational sac can be seen.
Human Chorionic Gonadotropin
Quantitative measurement of serum β-hCG is a very accurate method for determining gestational age in the first trimester of a normal pregnancy.26 This is extremely important in the diagnosis of ectopic pregnancy, because at the time of initial evaluation, many women will be unsure of their menstrual or conception dates; thus the exact gestational age is not known. The use of radioimmunoassay to measure serum β-hCG has greatly improved the time to obtain results as well as their accuracy.
Discriminatory Zone
An important factor when determining the viability and location of a pregnancy by vaginal ultrasound is the discriminatory zone. The discriminatory zone is defined as that level of β-hCG at which a normal singleton intrauterine pregnancy can be visualized on transvaginal ultrasonography.28 At most institutions, the discriminatory zone for a singleton pregnancy when using transvaginal ultrasonography is between 1500 and 2500mIU/mL (using the WHO Third International Standard, or International Reference Preparation).2
Serial β-hCG Determination
To distinguish a normal intrauterine pregnancy from a nonviable intrauterine or ectopic gestation, serial β-hCG determinations are performed. It is now well-established that the beta-hCG concentration rises almost linearly in the early weeks of a normally growing gestation, doubling every 1.4 to 2.1 days.27–29 Many clinicians rely on the rule of at least a 66% rise in β-hCG over 2 days based on earlier studies.27,30–33 More recent evidence suggests that the rise of β-hCG may be slower than previously reported, with 99% of all normal viable intrauterine pregnancies having an increase in β-hCG of at least 24% in 1 day and 53% in 2 days.34 Intervening when the 2-day rise in β-hCG is between 53% and 66% may result in the interruption of a viable pregnancy.
Uterine Cavity Sampling
In cases where gross products of conception (gestational sac or fetal parts) are not visible, verification of the presence of chorionic villi can be a problem, because final diagnosis with a permanent pathologic specimen takes up to 24 hours. One solution is to obtain a frozen section at the time of D&C, which has been shown to be very accurate in identifying products of conception, with almost no risk of false-positive results.35
Other techniques used to identify chorionic villi have not been found to be as sensitive. Floating the tissue obtained in saline solution will allow the trained gynecologist to identify villi in only 60% of cases where they can be identified histologically.36 The use of a stereomicroscope significantly improves the ability to identify chorionic villi, but is rarely available in common practice.37 Sampling of the uterine cavity with a pipelle biopsy instrument in an outpatient setting has been found to have relatively poor sensitivity of 30% to 63%.38,39 In the future, perhaps other forms of less invasive endometrial sampling, such as the handheld manual vacuum aspirator, will prove to have the necessary sensitivity for confirming products of conception.
Other Diagnostic Tests
Serum progesterone levels have also been used to aid in the diagnosis of ectopic pregnancy. Overall, serum progesterone levels are lower in ectopic pregnancies than in intrauterine pregnancies.40 Levels less than 5ng/mL are almost always (99.8%) associated with nonviable pregnancies, but these can be either abnormal intrauterine pregnancies (impending spontaneous abortion) or ectopic pregnancies.41 Conversely, progesterone levels of greater than 17.5ng/mL are rarely associated with ectopic pregnancies, with only 8% of ectopic pregnancies falling into this category.
Despite these strong correlations at either end of the concentration spectrum, serum progesterone levels have limited value in diagnosing ectopic pregnancies, because many patients’ values will fall between these extremes of values, where there is too much overlap to be discriminatory.42 In addition, serum progesterone levels are not readily available in many hospital laboratories on a “stat” basis, making the use of this test impractical in emergency situations.
Other laboratory tests evaluated for usefulness in the diagnosis of ectopic pregnancy include vascular endothelial growth factor (VEGF), CA-125, fetal fibronectin, and creatine kinase.43–49 Like serum progesterone, overlapping ranges of values for normal and abnormal pregnancies have prevented any of these markers from being useful in distinguishing ectopic and nonectopic gestations. Using genomics approaches, other promising serum protein markers have been identified that may ultimately prove to be discriminatory between intrauterine and ectopic pregnancies.50
Algorithm for Diagnosis
A simple diagnostic algorithm using ultrasound and serum β-hCG determinations can be helpful (Fig. 48-1).51 When a patient presents in early pregnancy with pain or uterine bleeding, the first step is transvaginal ultrasound. If a nonviable intrauterine pregnancy (e.g., impending spontaneous abortion) is visualized, standard management options are indicated based on symptomatology. Likewise, if an ectopic pregnancy is seen in the adnexa, treatment options are clear.
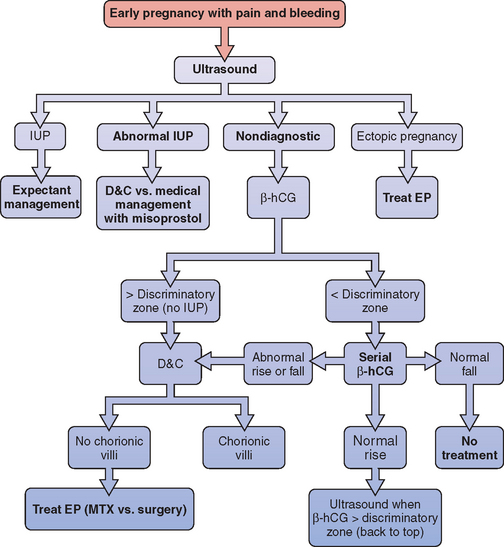
Figure 48-1 Diagnostic algorithm flow chart
(adapted from Gracia CR, Barnhart KT: Diagnosing ectopic pregnancy: A decision analysis comparing six strategies. Obstet Gynecol 97:464-470, 2001.)
In some cases where no chorionic villi are found on D&C, the clinical history is suggestive of a complete spontaneous abortion before evacuation, with heavy vaginal bleeding with passage of tissue and an open cervix. In these cases, it is appropriate to manage the patient expectantly with re-evaluation of serum β-hCG levels 12 to 24 hours after evacuation. If the β-hCG level drops sharply from preoperative levels, a complete spontaneous abortion is the most likely diagnosis, although a resolving ectopic pregnancy (sometimes referred to as a tubal abortion) is also possible. Keep in mind that 35% of women with an ectopic pregnancy are diagnosed when the β-hCG level is falling.28 If the β-hCG level plateaus or continues to rise, an ectopic pregnancy is highly likely, and immediate treatment should be instituted. This approach can also be used when the clinical suspicion of ectopic pregnancy is low, but no pathologist is available for intraoperative examination of the D&C specimen.
All patients for whom ectopic pregnancy was among the differential diagnoses should be followed with at least weekly β-hCG levels until β-hCG is no longer detectable in the serum. This may take up to several weeks, because a minimum decline in serial β-hCG concentration for a completed abortion ranges from 21% to 35% in 48 hours.52 A negative β-hCG value is the only sure way to confirm complete resolution of the ectopic pregnancy. There have been reports of tubal rupture with β-hCG levels as low as 5mIU/mL.53
Of all women with ectopic pregnancies who present with symptoms, about 50% will have β-hCG levels above the discriminatory zone and are therefore diagnosed within a single evaluation.3 The remaining 50% of women with ectopic pregnancies who seek medical attention will be found to have β-hCG levels below the discriminatory zone, and ultrasound is usually nondiagnostic. At this point in time, the sensitivity of transvaginal ultrasound for the diagnosis of intrauterine pregnancy, spontaneous miscarriage, and ectopic pregnancy has been shown to be only 25% to 33% and the predictive value is low.54
Screening Asymptomatic Patients
There may be some advantage to screening patients at high risk for ectopic pregnancy before the development of symptoms.55 Risk factors include previous history of ectopic pregnancy, tubal surgery, PID, sterilization, current IUD, and known tubal disease seen by hysterosalpingography or laparoscopy. In a study of 143 symptom-free women with these risk factors, screening was started before 7 weeks’ gestation with serial β-hCG measurements and ultrasound studies. In this particular study, 5.6% of the women were diagnosed with ectopic pregnancies. It is yet to be established that the potential benefits of this approach, including decreasing the risk of complications and patient reassurance, outweighed the drawbacks of false-positive diagnoses, increased costs, and increased emotional stress.56 For this reason, universal screening of women at increased risk for ectopic pregnancy cannot be recommended at this time.
TREATMENT
Laparotomy versus Laparoscopy
Laparotomy versus laparoscopy for the treatment of ectopic pregnancy has been compared in three prospective, randomized trials.57–59 Each concluded that the laparoscopic approach is superior to laparotomy. Laparoscopy resulted in less blood loss, less analgesia requirement, and a shorter duration of hospital stay compared to laparotomy. Laparoscopy was also found to be less costly in all three trials. Not surprisingly, a Cochrane review of the surgical treatment of ectopic pregnancy likewise concluded that laparoscopy is the treatment of choice for eligible patients.60
Exploratory Laparotomy: The Unstable Patient
Laparotomy via a pfannensteil incision will usually allow expeditious entry into the peritoneal cavity. On visualization of the pelvic structures, the site of implantation of the ectopic pregnancy should be immediately identified. A Kelly forceps (“clamp”) is then placed at the proximal portion of the fallopian tube, at the uterine cornu. This should virtually eliminate further blood loss, because most of the blood supply to the fallopian tube comes from branches of the uterine artery. A second Kelly clamp can then be placed along the mesosalpinx, meeting the end of the first clamp, so that all vessels within the mesosalpinx are occluded. Alternatively, a succession of Kelly clamps can be used to clamp the mesosalpinx as close to the tube as possible, as described by Damario and Rock.61 The entire tube and the ectopic gestation are then excised as one specimen. The pedicles are suture ligated with 2-0 or 3-0 vicryl or other synthetic absorbable suture. After assuring hemostasis, the pelvis should be evacuated of blood and clots, which can total up to several liters of blood loss (Table 48-2).
Table 48-2 Salpingectomy via Laparotomy or Laparoscopy — Surgical Steps
| Suprapubic Pfannensteil incision made for laparotomy |
| Fallopian tube elevated using Allis or Babcock clamp |
| Mesosalpinx clamped with succession of Kelly clamps or hemostats, just below the fallopian tube |
| Tube removed at site of uterine attachment close to cornua |
| Interrupted 2-0 or 3-0 delayed-absorbable suture (e.g., Vicryl) used for closure of pedicles |
| Inspection for hemostasis |
| or |
| Laparoscopic trocar ports placed (umbilical and at least 2 additional) for laparoscopic approach |
| Fallopian tube grasped and elevated distally with endo-grasper |
| Tube cauterized and then transected at cornual end, close to uterus |
| Specimen placed in endoscopic bag and removed via large port site |
Laparoscopic Approach
Salpingectomy
The surgical steps of laparoscopic salpingectomy are similar to those performed via laparotomy.56,62 Blood and clots present in the pelvis are removed with irrigation and suction so that the involved tube can be adequately visualized. The involved tube is grasped near the end where cutting will be initiated. If possible, the tube is resected starting proximally at the uterine cornu and continuing until the fimbrial end is reached. With less than ideal exposure, resection can proceed in the opposite direction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








