Fig. 15.1
Initial dissection required for Nissen’s closure. (Source: [13]. Reprinted with permission from Elsevier. © Elsevier 1991)
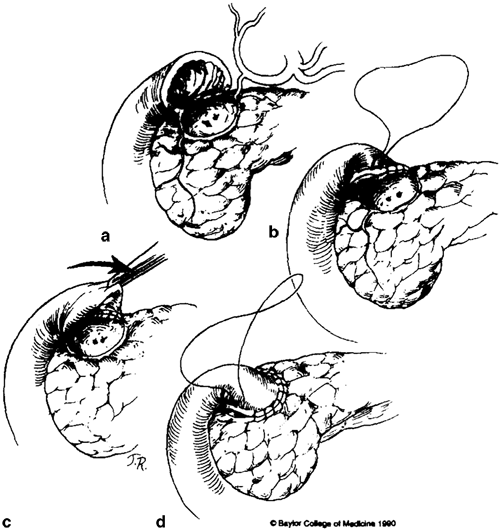
Fig. 15.2
Technique of Nissen’s closure for the difficult duodenum. (Source: [13]. Reprinted with permission from Elsevier. © Elsevier 1991)
Bancroft Technique
Bancroft’s closure, first described in 1932, begins with a submuscular dissection starting in the distal antrum and progressing toward the duodenum [13]. The stomach is then divided, and dissection is carried down around the circumference of the antrum distally. Once the antral mucosa and submucosa have been dissected in their entirety to the duodenal junction, a frozen section confirms the presence of duodenal mucosa and this is then closed. Following mucosal approximation, the excess antral muscle tissue is trimmed and closure is completed with a running nonabsorbable suture line (see Fig. 15.3). While Bancroft’s closure can be a useful strategy in the management of a difficult duodenum during distal gastrectomy, this approach must be planned for early in the operation. As the technique relies on viable tissue around the distal antrum, the right gastric and gastroepiploic arteries, which are typically ligated at the beginning of the dissection, must instead be preserved.
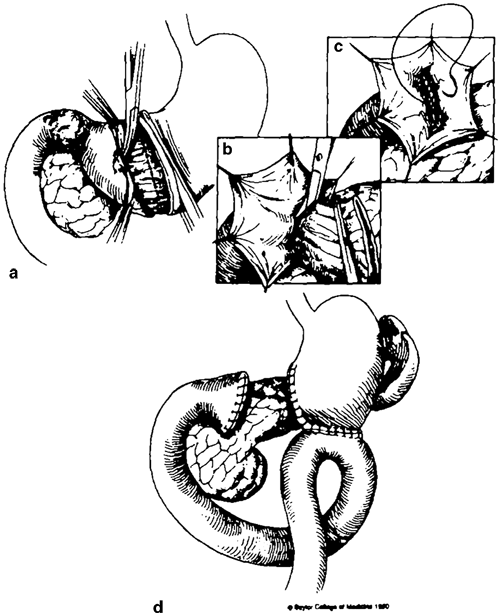

Fig. 15.3
Bancroft’s closure. (Source: [13]. Reprinted with permission from Elsevier. © Elsevier 1991)
Tube Duodenostomy and Drainage
Drainage of the duodenal stump by way of catheterization has long been championed as a potential means of reducing the risk of blowout, albeit with some controversy. As early as the late 1880s, both Billroth and von Langenbuch had reported on the use of indwelling duodenal catheters for the purpose of postoperative feeding. Not until more than 20 years later, however, did Neumann describe catheter duodenostomy for stump decompression [14]. In the 1950s, tube duodenostomy was revisited by Welch, and by the 1970s had gained traction as an acceptable and established approach in managing the difficult duodenum [15–17].
Early experiences using tube duodenostomy were plagued with reports of leakage and no improvement in patient outcomes, and the technique was initially met with skepticism. Over time, results improved, but the technique was nonetheless slow to gain considerable popularity. To this day, there remains considerable controversy regarding whether tube duodenostomy is an appropriate and helpful procedure, and we advocate for selective use based on surgeon preference and individual circumstances.
While lateral T-tube catheter drainage of the duodenal stump has been described and used with success [10], contemporary approaches to tube duodenostomy typically involve introduction of a Foley, Pezzer, or straight catheter via the stump of the duodenum to approximately 5 cm (see Fig. 15.4) [18]. The open end of the duodenum is then gently secured in place around the tube using a pursestring 3-0 absorbable suture, taking care to slightly invaginate the suture into the lumen of the duodenum (see Fig. 15.5). The seal around the catheter should then be tested by injecting 30–60 ml of sterile saline into the duodenum via the tube and observing for leaks. Once an adequate seal has been established, an omental pedicle is brought into place at the point of tube entry and secured in place. The distal end of the tube is then brought through the abdominal wall, minimizing the length of the intraabdominal portion of the tube.
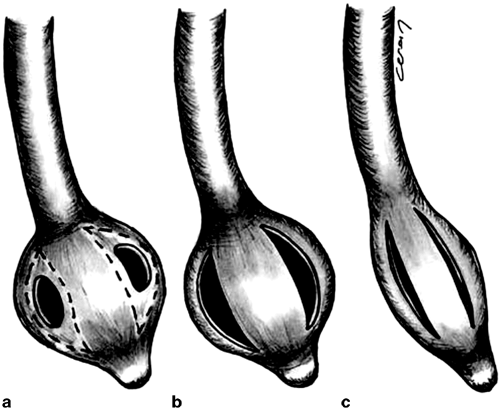
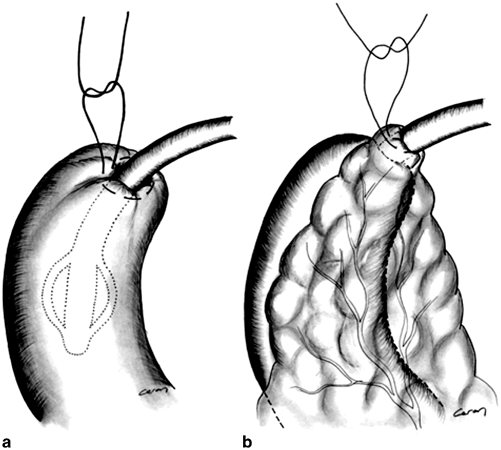

Fig. 15.4
Modification of a standard Pezzer-type catheter for use in tube duodenostomy: a Original appearance of the tube. b Final appearance before inserting into the duodenum. c Appearance of the tube while removing. Note less traumatic effect of the tube to the duodenal stump. (Source: [18]. Reprinted with permission from Springer. © Springer Science and Business Media 2007)

Fig. 15.5
a Tube duodenostomy through the duodenal stump. b The duodenal stump with Pezzer drain in it has been protected by surrounding omentum. (Source: [18]. Reprinted with permission from Springer. © Springer Science and Business Media 2007)
Management of Stump Blowout
The management of a patient with a duodenal stump leak is one of the most challenging clinical scenarios faced by gastrointestinal surgeons. Historically, duodenal stump leak has been characterized by significant morbidity and mortality. While this has improved over time, even in the current era this condition can be associated with substantial mortality. Successful management of duodenal stump leak requires a comprehensive approach that incorporates optimal medical management, judicious employment of percutaneous radiologic procedures, and sound clinical decision-making regarding the need for reoperation, timing, and surgical approach.
Medical Management
Optimizing medical therapy greatly enhances the likelihood of successful treatment of duodenal stump leak. A thorough evaluation of the patient’s clinical condition is a critical first step. Clinicians must recognize that these patients may decompensate rapidly, and patients who display signs of hemodynamic instability or sepsis should be transferred to an intensive care setting. Appropriate intravenous access should be ensured. Patients will frequently require central line placement for the administration of vasoactive agents, monitoring of central venous pressure, and administration of total parenteral nutrition (TPN). Adjuncts such as arterial line placement may also be necessary for close hemodynamic monitoring. Along with these basic steps to resuscitate the patient and restore euvolemia, the initiation of broad-spectrum antibiotics is required. Initial antibiotic selection is generally broad spectrum and includes coverage of Gram-negative and anaerobic organisms. Antifungal coverage may be necessary in patients who display signs of sepsis in the context of previous treatment with a prolonged course of antibiotics.
Another important consideration for successful management is optimization of nutritional status. Patients with duodenal stump leak are commonly malnourished and require additional caloric intake secondary to the considerable physiologic stress associated with this condition. Due to the compromised state of the upper gastrointestinal tract, oral feeding is generally not possible. If enteral access is available in the form of a feeding jejunostomy tube, enteral nutrition is preferred, but this is commonly not the case. Thus, for most patients, initiation of TPN is common to provide adequate nutrition in this setting. Nutritional parameters including prealbumin, transferrin, and albumin should be monitored at least weekly, and adjustments to TPN administration made accordingly.
Other adjunctive medical therapies are also commonly administered in patients with duodenal stump leak. Gastrointestinal prophylaxis with proton pump inhibitors or histamine blockers may combat stress gastritis. Additionally, administration of the somatostatin analogue octreotide may be employed in an effort to reduce the volume of effluent from the duodenal stump and promote fistula closure.
Percutaneous Radiologic Techniques
With significant advances in imaging technology and greater sophistication of image-guided percutaneous techniques in the current era, radiologic intervention has become the mainstay of therapy for duodenal stump leak. The first requirement of successful management is establishing control of abdominal sepsis. This can frequently be achieved by percutaneous drainage of intraabdominal fluid collections with catheter placement to allow ongoing evacuation of fluid. The goal of this intervention is to completely drain intraabdominal fluid and convert the duodenal leak into a stable duodenal fistula. This may require an aggressive approach with placement of multiple catheters and frequent trips to radiology suite for catheter repositioning and upsizing to gain optimal control of intraabdominal fluid.
After initial control of abdominal sepsis and successful establishment of a duodenal fistula, treatment strategies shift to interventions with the goal of achieving closure of the duodenal fistula. A common technique employed to decrease fistula output is biliary diversion by percutaneous transhepatic biliary drainage. The goal of this procedure is to divert the majority of bile flow away from the duodenum and thus significantly reduce the volume of effluent from the duodenal stump. In a small study by Zarzour and colleagues, percutaneous biliary drainage significantly reduce fistula volume from a mean value to 775 ml to less than 50 ml and lead to fistula closure in five of six patients [19]. Some centers have expanded on this technique by adding placement of a biliary occlusion balloon in addition to percutaneous biliary drainage in order to completely divert all bile flow [20].
Another percutaneous strategy for managing duodenal stump leak is percutaneous placement of tube duodenostomy. In a recent report, Oh and colleagues describe a staged approach for establishing tube duodenostomy using a Foley catheter [21]. In the initial phase of this technique, a percutaneous pigtail catheter is placed to drain duodenal stump effluent and establish a fistulous tract. After establishment of a fistulous tract, the Foley catheter is then advanced through the tract directly into the duodenum and confirmed via fluoroscopy.
The Decision to Operate and Surgical Approach
Decision-making regarding the need for reoperation and timing of such intervention in patients with duodenal stump leak is complex and requires mature surgical judgment. In the first 2–4 weeks following the index procedure, there is a great degree of inflammation in the dissection field, making reoperative surgery difficult and potentially hazardous. In light of these considerations, many surgeons prefer an initial trial of percutaneous management as described above to temporize the situation, control abdominal sepsis, and allow patient stabilization.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








