CHAPTER 117 Diverticular Disease of the Colon
The earliest description of the pathology of diverticulosis traditionally has been attributed to Cruveilhier in 1849, although an earlier description by Sir Erasmus Wilson was noted in an editorial comment in Lancet in 1840.1 Occasional reports of the condition appear in the literature of the 19th century. The role of surgery in the treatment of acute diverticulitis was discussed by Mayo and associates in 1907. The presence of uncomplicated pseudodiverticula, herniations of the mucosa and submucosa through the muscularis of the colon, was defined as diverticulosis on radiologic studies by Case in 1914. The role of diet in the pathogenesis of diverticulosis was advanced in the landmark paper by Painter and Burkitt in 1971.2 Our knowledge of the epidemiology and clinical behavior of diverticular disease has grown rapidly over the past few decades, in large measure because of advances in imaging technology.
The incidence of this disorder is increasing in the Western world.3–5 In 1998, diverticular disease resulted in total medical costs of $2.499 billion (direct costs: $2.358 billion; indirect costs: $141 million) and accounted for 230,058 hospital stays (with diverticular disease as the primary diagnosis only), 147,785 outpatient hospital visits, 165,343 emergency department visits, and 2,216,519 physician office visits.6
EPIDEMIOLOGY
Because most patients are asymptomatic, the true incidence and prevalence of diverticulosis are difficult to ascertain. Autopsy series can underestimate prevalence if small diverticula are missed or not commented upon by the pathologist, whereas series using barium enema for diagnosis can overestimate the condition and lead to selection bias because the study usually is done to investigate symptoms.7 Recent studies report overall prevalence rates for diverticulosis of 12% to 49%.8 The prevalence of diverticular disease clearly increases with age, ranging from less than 10% in those younger than 40 years of age to an estimated 50% to 66% of patients 80 years of age and older.9 Diverticulosis appears to be just as common in men and women,10 although men may have a higher incidence of diverticular bleeding and women may have more episodes of obstruction or stricture.11
Diverticulosis, with its striking geographic variation, has been termed a disease of Western civilization. The disorder is extraordinarily rare in rural Africa and Asia; conversely, the highest prevalence rates are seen in the United States, Europe, and Australia.2 Within a given country, the prevalence of colonic diverticula also can vary among ethnic groups. In Singapore, the annual incidence of diverticulitis in Chinese inhabitants was 0.14 cases per million, whereas in European inhabitants, the rate was 5.41 per million.12 Japanese-born persons who migrated to and lived in Hawaii had diverticulosis at autopsy in 52% of cases—much more frequently than those remaining in Japan.13 A registry of residents of Sweden revealed that immigrants to that country from low-prevalence regions, including Asia, Africa, and the Middle East, had hospitalization rates for diverticular disease that were 30% to 50% lower than those of Swedish natives and immigrants from Western countries.14 The magnitude of this gap narrowed as time from immigration to Sweden increased, however, and the gap nearly disappeared after 10 or more years in the country.
As an individual country becomes more urbanized, an increase in diverticulosis seems to follow over time, as has been shown in Singapore and Israel.15,16 This observation may be attributable in part to a Westernization of diet with an increase in meat ingestion and a diminution of fiber intake as a country becomes more industrialized.10 Aside from age, geography, and ethnicity, other inherited and acquired risk factors have been associated with the presence of diverticulosis (Table 117-1). The role of dietary fiber is detailed later in this chapter. There is no conclusive evidence that diverticular disease is associated with colorectal cancer.
Table 117-1 Factors That Influence the Risk for Diverticulosis
| Increased Risk |
| Decreased Risk |
| Equivocal or No Risk |
Much of the sentinel data on the natural history of diverticulosis was reported by Parks in Belfast in the 1960s and 1970s,9,17 although these data suffer from the selection bias of studying only symptomatic patients. Parks observed that patients with many diverticula were, on average, older than those with few diverticula, suggesting that the number of diverticula in a patient increases with age. In contrast, patients with total colonic involvement were, on average, younger than those with segmental disease, suggesting that the pattern of colon involvement may be determined early on and remain more or less fixed. A study of barium enemas performed an average of 4.4 years apart in patients with diverticulosis demonstrated no apparent progression of disease in most patients.18
PATHOLOGIC ANATOMY
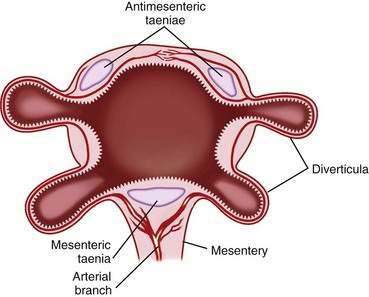
Diverticula do not arise randomly around the circumference of the colon. Rather, they originate in four distinct rows that correspond to the four sites of penetration of the bowel wall by the major branches of the vasa recta: on either side of the taenia mesocolica and on the mesenteric side of the taenia omentalis and taenia libera (Fig. 117-1). The diverticula point to the mesenteric border, and no bona fide diverticula arise from the antimesenteric intertaenial area. Diverticula maintain this fixed anatomic relationship to the taenia and are conspicuously absent from the portion of colon between the two antimesenteric taenia. Because diverticula do not involve all the layers of the colon wall, but rather are herniations of the mucosa and submucosa through a defect in the muscularis, colonic diverticula are, strictly speaking, pseudodiverticula. In this chapter, the incorrect, but traditional, terms diverticulum (singular) and diverticula (plural) are used rather than pseudodiverticulum and pseudodiverticula.
Diverticula can vary in number from one to literally hundreds. The typical size of a diverticulum is 3 to 10 mm in diameter, but they can exceed 2 cm. Giant colonic diverticula have been described, with sizes up to 25 cm. Most giant diverticula are discovered incidentally, are single, are located in the sigmoid colon, and are asymptomatic. Rarely, giant sigmoid diverticula exhibit a valve-like phenomenon by which gas can enter but not leave; they get progressively larger over a relatively short period of time, can obstruct or perforate, and thereby require surgery.19
Diverticula can occur anywhere in the colon, and the segment typically involved depends highly on geography. In Western countries, diverticula occur mainly in the left colon, with up to 90% of patients having involvement of the sigmoid; only 15% have right-sided with or without left-sided involvement.1,9,20 In contrast, right-sided involvement is predominant in Asian countries. The ascending colon is involved in about 75% of patients in Singapore15,21 and only 25% of patients have sigmoid disease. In Japan, the prevalence of right-sided diverticulosis found on barium enema doubled (from 10% to 20%) from 1982-1997, a period over which left colon involvement remained the same (∼4%).22 The structural morphology of the diverticula found on either side of the colon appears to be identical worldwide. Although the precise factors causing the segmental predominance of left colon and right colon involvement in the West and East, respectively, are not known, environmental (e.g., dietary) and genetic factors are believed to play roles. Additionally, a blending of genetic and cultural lineages could explain why many people in the United States develop pancolonic diverticulosis.
ETIOLOGY AND PATHOGENESIS
COLONIC WALL STRUCTURE
Electron microscopic studies confirm that the colonic walls in patients with diverticulosis have structurally normal muscle cells but, compared with controls, contain a greater than 200% increase in elastin deposition between the muscle cells in the taenia.23 The elastin is laid down in a contracted form, resulting in shortening of the taenia and bunching of the circular muscle. An increase in type III collagen synthesis in patients with diverticulosis also has been described, raising the possibility that age-related changes in collagen composition play an etiologic role.24
In addition to an overall increase in the collagen content, an overexpression of a tissue inhibitor of metalloproteinases has been identified in colons with diverticula.25,26 Because matrix metalloproteinases are believed to regulate deposition of extracellular matrix proteins, an increase in their regulatory molecule, tissue inhibitor, might explain the increase in elastin and collagen deposition found in diverticular colons. The importance of intestinal wall connective tissue also is underscored by the higher rate of diverticulosis reported in patients with connective tissue disorders, such as Ehlers-Danlos syndrome, Marfan’s syndrome, and scleroderma.5
MOTILITY
Early investigations using colonic manometry demonstrated higher resting, postprandial, and neostigmine-stimulated luminal pressures in patients with diverticulosis compared with controls.27,28 Based on simultaneous manometry and cineradiography, Painter proposed a theory of segmentation, postulating that contraction of the colon at haustral folds caused the colon to act not as a continuous tube but as a series of discrete “little bladders,” which led to excessively high pressures within each segment.28,29 He further suggested that the Western diet might alter colonic motility to augment hypersegmentation, thereby increasing the tendency to form diverticula.
More recently, using flexible endoscopy to accurately place manometric catheters within the sigmoid colon, the motility abnormalities previously described have been confirmed.30 Further, patients with symptomatic diverticular disease have been reported to have higher motility indices than either asymptomatic patients or persons without diverticula.31 In addition to increased contraction amplitude, another study found retropropagation of contractile waves in diverticular segments of colon, indicating that motility in these patients may be abnormal in magnitude and direction.32 An Asian study has confirmed elevated resting and stimulated luminal pressures in the presence of proximal colon diverticulosis.33
Although the demonstration of abnormal motility and elevated intraluminal pressures in the diverticular colon has been consistent, the physiologic basis for these abnormalities is less clear. Ion transport across the epithelial membrane of diverticular colons is the same as in controls.34 The number of myenteric and submucosal plexus neurons in diverticular colons are normal; however, the number of interstitial cells of Cajal (enteric pacemaker cells) is reduced.35 Increased activity of excitatory cholinergic nerves and a decreased activity of nonadrenergic, noncholinergic inhibitory nerves, the latter in part mediated by nitric oxide, have been demonstrated in diverticular colons compared with control colons.36 The magnitude of electrically stimulated contraction in diverticulosis-affected sigmoid colon is markedly reduced by antagonists of cholinergic and tachykinin neurotransmitters.37 In contrast, a study of the tachykinin neurotransmitter system showed a decreased contractility of circular muscle induced by substance P in diverticular colons compared with normal ones.38
DIETARY FIBER
The wide geographic variation of diverticular disease, with higher prevalence in countries with a Westernized diet, has long suggested a dietary factor in its pathogenesis. Low intake of dietary fiber has been strongly suspected to be the main dietary factor behind these geographic differences. Burkitt and Painter were early proponents of this theory, labeling diverticulosis a “deficiency disease” that, like scurvy, should be avoidable with dietary changes.2 In one important study, they demonstrated that persons in the United Kingdom who consumed a refined, low-fiber Western diet had stool transit times more than twice as slow and stool weights significantly less than those of rural Ugandans eating a diet very high in fiber.39 The long intestinal transit time and smaller-volume stools were believed to allow an increase in intraluminal pressure, thus predisposing to diverticular herniation, whereas bulkier stools were associated with less colonic contraction and lower wall pressures.
Although more recent studies in Western cohorts have failed to confirm this finding in humans consistently, corroborative animal data do exist. Wistar rats fed low-fiber diets developed diverticula in 45% of instances, compared with only 9% of those on high-fiber diets.40 In humans, other observational evidence exists with respect to the etiologic role of fiber in diverticulosis. In the United States, fiber intake decreased by 28% from 1909 to 1975,5 a period of dramatic increase in the prevalence of diverticular disease. In a British study, a group of vegetarians on a high-fiber diet had a lower prevalence of diverticulosis than did nonvegetarians (12% vs. 33%).41
Dietary influences for diverticulosis may have different effects on the right and left sides of the colon. Right-sided diverticular disease was shown in an Asian study to have no association with intake of fruits and vegetables or supplemental fiber, but it was strongly associated with meat consumption.42 Whether these associations apply to Westerners with right-sided diverticulosis is not known.
UNCOMPLICATED DIVERTICULOSIS
ASYMPTOMATIC DIVERTICULOSIS
A possible prophylactic benefit of a high-fiber diet has been suggested in two publications of 47,888 male health professionals who were followed over four years and in whom 385 (0.75%) new cases of symptomatic diverticular disease were identified.43,44 A dietary review found an inverse association between dietary fiber intake and the risk of subsequently developing clinically evident diverticular disease. The study also noted that fruit and vegetable fiber, or insoluble fiber, had a greater protective effect than did cereal fiber. Conversely, diets high in fat and red meat were associated with an increased risk of diverticular disease. Although prospective randomized trials are lacking, these findings suggest that patients with asymptomatic diverticulosis and SUDD (see later) might benefit from increasing their fruit and vegetable fiber intake while decreasing their fat and red meat consumption, a sensible lifestyle change that likely also provides other salutary health benefits.
For decades, it has been a widely held but weakly supported belief that patients with diverticulosis should avoid consumption of seeds, nuts, and popcorn to avoid possible plugging of diverticula and precipitating an episode of diverticulitis; this theory also was investigated in the aforementioned cohort of male health professionals.45 Not only was no increase in risk of diverticular complications found as a result of consuming nuts, corn, popcorn, or seeded fruit (strawberries or blueberries), but consumption of nuts and popcorn were inversely associated with the risk of developing diverticulitis. Although there probably is not enough evidence to warrant actively encouraging patients with diverticulosis to eat large quantities of nuts, popcorn, or seeds, neither should these foods be categorically avoided.
SYMPTOMATIC UNCOMPLICATED DIVERTICULAR DISEASE
Clinical Features
Some patients come to clinical attention because of nonspecific abdominal complaints and are found to have diverticulosis coli. A causal relationship between the diverticulosis and the abdominal symptoms often is difficult to establish. If there are features that are consistent with a diverticular source, however, and there is no evidence of a serious inflammatory condition, the disease may be defined as SUDD. Most patients with SUDD present with left lower quadrant pain; the British refer to this condition as painful diverticular disease. The pain often is exacerbated by eating and diminished by defecation or the passage of flatus. Patients also may report other symptoms of colonic dysfunction, including bloating, constipation, diarrhea, or the passage of mucus per rectum. Physical examination may be normal or may reveal fullness or mild tenderness in the left lower quadrant, but frank rebound or guarding is absent. Because rates of occult bleeding in diverticulosis are similar to those in healthy controls, a positive fecal occult blood test never should be attributed to diverticulosis.46
Because a barium enema can characterize the number, size, and location of diverticula, it previously had been a commonly used initial study in such patients. Barium enema, however, may be insufficient to rule out competing or associated diagnoses such as malignancy in patients with diverticulosis. In symptomatic patients in whom a double-contrast barium enema showed sigmoid diverticulosis, subsequent colonoscopy confirmed only 55% of neoplastic lesions that were suspected on the barium enema, whereas eight polyps and three malignancies were identified on colonoscopy that were missed on the barium enema (24% false-negative rate for barium enema).47
Although the barium enema may remain useful in certain cases, particularly if a colonoscopy cannot be performed safely or completely, endoscopic evaluation (Fig. 117-2) has assumed a primary role in the evaluation of most patients, particularly to exclude neoplasia. It once was believed to be unsafe to perform colonoscopy in patients with diverticulosis because of an increased risk of perforation; however, it subsequently was demonstrated that manometrically measured burst pressures for diverticula far exceed the usual pressures encountered during routine sigmoidoscopy or colonoscopy, even with the endoscope pressing against the wall or with heavy air insufflation.48 These data and many years of clinical experience have demonstrated the relative safety of using endoscopy to evaluate patients with abdominal symptoms. Caution should be exercised in patients with suspected or proved diverticulitis, however, because of a theoretical risk of perforating the wall of a diverticulum that has lost its integrity due to inflammation. In all patients, air insufflation should be minimized and excessive force in advancing the endoscope should be avoided.
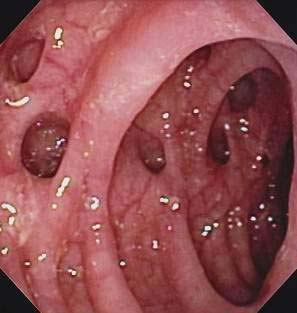
Figure 117-2. Colonoscopic view of the sigmoid colon in a patient with symptomatic uncomplicated diverticular disease.
The diverticula-laden colon can be challenging for the endoscopist to navigate because of spasm, luminal narrowing, fixation from prior inflammation and fibrosis, or confusion between luminal and diverticular openings. A number of solutions have been proposed to alleviate this problem. The use of a smaller-diameter pediatric colonoscope can be useful for difficult colons. One group has reported a success rate of more than 90% with a pediatric colonoscope in cases in which an adult colonoscope could not be passed through the sigmoid; 44% of these patients had diverticulosis.49 When colonoscopy with standard and pediatric colonoscopes were compared, the reason for failure to complete the examination was thought to be stenosing diverticular disease in 12 of 14 patients with the standard colonoscope, compared with two of eight with the pediatric colonoscope.50 A technique involving distention of the lumen with 100 to 300 mL of water, called the sigmoid floatation maneuver, was said to have facilitated colonoscopy in six technically difficult cases of severe diverticular disease.51
Occasionally, an endoscopist encounters an inverted diverticulum, where a diverticular dome protrudes into the lumen instead of out from it. These inversions often resemble polyps endoscopically, although they may be distinguished by their normal overlying mucosa, broad base, and location within a bed or row of diverticula. They are soft-appearing when manipulated with the endoscope tip or a biopsy forceps (pillow sign) and may be reducible. On barium enema, inverted diverticula appear as broad-based sessile polyps with a characteristic central umbilication,52 although it is not always possible to distinguish such a diverticulum from a polyp. When inverted diverticula are encountered, their removal should be avoided, although inadvertent colonoscopic diverticulectomy has been reported,53 and these patients had uneventful recoveries with conservative therapy.
The presenting symptoms of SUDD overlap considerably with those of irritable bowel syndrome (IBS). Some authorities have postulated that diverticula are, in fact, a late consequence of IBS. In a Danish cohort of IBS patients, one third of whom had diverticula, no difference in symptoms or prognosis was detected between those with diverticula and those without diverticula over more than five years of follow-up.54 This finding led the investigators to conclude that there is no basis to consider SUDD as a separate entity from IBS. Ritchie reported that there was a similarity of pain sensation from rectal balloon distention in patients with IBS and those with diverticulosis.55 Whether these two disorders are distinct entities is unknown and probably not clinically important, because both are treated in a similar nonspecific fashion with equally good prognoses.
Treatment
Fiber
Aside from the reported preventive effect of dietary fiber described earlier, fiber also is a mainstay of treatment for SUDD. Many uncontrolled trials of fiber in SUDD have been reported, all limited by a high placebo response rate. A randomized, double-blind trial from Oxford University showed a statistically significant decrease in bowel symptoms relative to controls in patients with SUDD who were placed on a high-fiber diet56; the separation between treatment and control groups, however, was not noted until the three-month follow-up evaluation. It is important to instruct patients to start fiber supplementation at a low dose and slowly increase the dose, because patients initially can worsen from diarrhea, gas, or bloating if the fiber dose is started too high or quickly. Because it often can take months to improve, as demonstrated in the Oxford study, the initial adverse symptoms can discourage patients from adhering to the fiber supplements if they are not counseled properly. In contrast, another study of fiber supplementation in patients with diverticular disease showed no significant improvement in overall symptoms, although decreased transit time and increased stool frequency were documented.57 Despite these conflicting data and the certainty that diverticula do not regress with an increased fiber intake, some amelioration of symptoms in patients with SUDD often can be seen with a high-fiber diet.
5-Aminosalicylic Acid
Additional medical therapies for SUDD have been studied since 2000, drawing on approaches that are effective in other colonic diseases such as inflammatory bowel disease (IBD) and IBS. 5-Aminosalicylate (5-ASA) compounds, a well established therapy for ulcerative colitis and Crohn’s disease, have been evaluated as a potential treatment for SUDD. Although patients with SUDD by definition lack severe or overt inflammation, in some patients subtle inflammation is suspected even without gross signs of diverticulitis; it is these patients that the anti-inflammatory properties of 5-ASA might benefit. Published studies that have examined the role of 5-ASA were randomized to either daily or cycled (e.g., 10 days per month) 5-ASA but were not placebo controlled.58–60 Results of each study show a significantly reduced symptom score relative to pretreatment scores, but the lack of a control arm and the known high placebo-response rates in functional bowel syndromes make the results difficult to interpret. The doses of 5-ASAs used in these studies were lower than generally are used for IBD. These results, combined with the relative safety of this medication class, make 5-ASAs a promising therapy for SUDD, although placebo-controlled trials supporting its efficacy will be needed before widespread use can be advised.
Antibiotics and Probiotics
The role of pathogenic and nonpathogenic bacteria in intestinal disease is being increasingly scrutinized. Some have postulated that a disturbance in the local microflora in and around diverticula61 might predispose to diverticulitis. If this were true, medications that alter this flora might help treat or prevent attacks of diverticulitis.
In contrast, rifaximin, a nonabsorbable antibiotic with broad-spectrum activity, mitigates some of these concerns by its solely luminal activity and by its use as an emerging potential therapy for C. difficile infection. Rifaximin has been studied in patients with SUDD and has shown promise in reducing frequency and severity of symptoms.62,63 Rifaximin has also been shown to be effective for IBS,64 possibly by eradicating concomitant small bowel bacterial overgrowth (SIBO). Whether inadvertently treating SIBO is the actual reason for the effectiveness of rifaximin in SUDD is not known.
Based on the same theory that altered local microflora is present in these patients, probiotics also have been studied in SUDD.59,65 Although some benefit has been shown, such trials are small and lack a placebo group. Although higher-quality evidence needs to be produced to support this approach, the microflora may become an important target for therapy in SUDD in the coming years; a number of trials are under way evaluating anti-inflammatory agents or probiotics.
Role of Surgery
Surgical intervention generally is not considered for patients with truly uncomplicated diverticulosis, because the risks of surgery outweigh the benefits in most cases. Some patients with subclinical or smoldering diverticulitis present with pain characteristic of diverticulitis but show no signs of systemic inflammation, such as fever or leukocytosis. In a cohort of such patients from the Mayo Clinic who underwent sigmoid resection with primary anastomosis for their symptoms without signs or laboratory markers of systemic inflammation, 76% of the resected specimens had evidence of acute or chronic diverticular inflammation.66 Sigmoid colectomy in these patients resulted in resolution of pain in 88% and complete resolution of symptoms in 76% after one year or more of follow-up. This finding underscores the importance of clinical follow-up and an open mind regarding patients with apparently uncomplicated disease whose symptoms do not improve with conservative treatment.
COMPLICATED DIVERTICULOSIS
Diverticulitis, defined as inflammation, infection, or both, associated with diverticula, is probably the most common clinical manifestation of this disorder, affecting an estimated 10% to 25% of patients with diverticula.9 It generally is believed to be the result of perforation of a single diverticulum.67 When this results in a localized phlegmon, the term uncomplicated diverticulitis is used. Complicated diverticulitis refers to cases associated with abscess, free perforation with peritonitis, fistula, or obstruction.68 Besides diverticulitis, the other major form of complicated diverticular disease is bleeding, which is discussed later in this chapter.
UNCOMPLICATED DIVERTICULITIS
Pathophysiology
The process by which a diverticulum becomes inflamed has been likened to that causing appendicitis, in which the diverticular sac becomes obstructed by inspissated stool in its neck; the fecalith abrades the mucosa of the sac, causing low-grade inflammation and further blocking drainage. Histologically, one of the earliest signs of inflammation is hyperplasia of the mucosal lymphoid tissue, with lymphoid tissue aggregation at the apex of the involved sac.69 The obstructed diverticulum predisposes to expansion of the normal bacterial flora, diminished venous outflow with localized ischemia, and altered mucosal defense mechanisms. One such alteration is a defective CD2 pathway-induced apoptosis, which has been found in lamina propria lymphocytes in patients with diverticulitis, possibly leading to an up-regulation of the local immune response in these patients similar to that seen in patients with IBD.70 Evidence also suggests that cytomegalovirus (CMV) reactivation might contribute to local inflammatory activity, because active CMV replication was found in tissue from the affected bowel segments of more than two thirds of patients with diverticulitis.71
The cascade of events initiated by fecalith obstruction, and possibly enhanced by underlying innate or acquired abnormalities, allows bacteria to breach the mucosa and extend the process transmurally, ultimately leading to perforation.72 The extent and localization of the perforation determine its clinical behavior. Microperforations can remain very well localized, contained by the pericolic fat and mesentery, and cause small pericolic abscesses. A larger perforation can allow a more extensive abscess to form, which can track longitudinally around the bowel wall. This process can lead to a large inflammatory mass, fibrosis, extension to other organs, or fistula formation. Free perforation into the peritoneum causing frank bacterial or fecal peritonitis can be life threatening, but fortunately it is uncommon, with a population incidence of 4 cases per 100,000 population per year.3,73 Hinchey and associates have described a staged grading system reflecting the severity of perforation (Table 117-2).74
Table 117-2 Hinchey Classification of Colonic Diverticular Perforation
| STAGE | DEFINITION |
|---|---|
| I | Confined pericolic abscess |
| II | Distant abscess (retroperitoneal or pelvic) |
| III | Generalized peritonitis caused by rupture of a pericolic or pelvic abscess (not communicating with the colonic lumen because of obliteration of the diverticular neck by inflammation) |
| IV | Fecal peritonitis caused by free perforation of a diverticulum (communicating with the colonic lumen) |
Clinical Features
Patients with acute diverticulitis typically present with left lower quadrant abdominal pain, reflecting the propensity for this disorder to occur in the sigmoid colon in Western countries; a redundant sigmoid colon, however, can manifest with suprapubic or right-sided pain. In contrast, Asian patients with diverticulitis have predominantly right-sided symptoms, corresponding to the location of their diverticula.75 The pain may be intermittent or constant and frequently is associated with a change in bowel habits, either diarrhea or constipation.76 Anorexia, nausea, and vomiting also can occur. Dysuria and urinary frequency can result from bladder irritation caused by the adjacent inflamed sigmoid colon.
Physical examination usually discloses localized tenderness, generally in the left lower quadrant; however, as noted, right-sided signs do not preclude the possibility of diverticulitis. Guarding and rebound tenderness may be present, as may a tender, cylindrical, palpable mass. Bowel sounds typically are depressed but may be normal in mild cases or increased in the presence of obstruction. Rectal examination can disclose tenderness or a mass, particularly with a low-lying pelvic abscess. Fever is present in most patients, whereas hypotension and shock are unusual. The white blood cell (WBC) count commonly is elevated, although one study reported a normal WBC count with no left shift in 46% of patients.76 No other laboratory abnormalities are routinely helpful, although they can help to rule out other diagnostic possibilities in select patients.
The differential diagnosis for diverticulitis is extensive. Acute appendicitis is the misdiagnosis most often made in patients with diverticulitis, particularly with right-sided disease. In Hong Kong, where awareness of the predominance of right-sided diverticulosis presumably is high, 34 of 35 patients with right-sided diverticulitis initially were believed to have acute appendicitis.75 Although appendicitis is, on average, a disease of younger patients than is acute diverticulitis, there is a wide range of ages for both. Clinical suspicion for one must remain high when diagnosing the other on clinical grounds. Other common diagnoses that need to be considered include IBD; other forms of colitis (infectious or ischemic); colorectal cancer; and gynecologic conditions such as pelvic inflammatory disease, ovarian cyst rupture, and ovarian torsion. Occasionally, diverticulitis can occur concomitantly with other diseases; in one study of patients admitted to the hospital with diverticulitis, 7% were found later also to have a colon malignancy.77
Diagnosis
Most patients with acute diverticulitis present with signs and symptoms sufficient to justify the clinical diagnosis and institution of empiric therapy. Clinical diagnosis can, however, occasionally be inaccurate, and emergency surgery for presumed diverticulitis, without the benefit of radiologic confirmation, carries a misdiagnosis rate as high as 34% to 67%.78 Therefore, radiologic studies to confirm the diagnosis of diverticulitis should be employed, particularly if invasive intervention may be required.
Plain Films
An erect chest film, together with erect and supine abdominal films, should be performed on patients with significant abdominal pain. The erect chest film has the dual purpose of detecting pneumoperitoneum, which has been reported to be present in up to 11% of patients with acute diverticulitis,79 and of assessing cardiopulmonary status in a generally elderly population with common comorbid illness. Plain abdominal films are abnormal in 30% to 50% of patients with acute diverticulitis,79,80 with findings that include bowel dilatation from obstruction or ileus, or a soft tissue density suggesting an abscess.
Contrast Enema Examinations
Contrast barium enema (Fig. 117-3) had been the diagnostic standard for diverticulitis and its complications for many years. Because the use of barium in the setting of an intestinal perforation carries a risk of barium peritonitis, only water-soluble contrast enemas, such as Gastrografin, should be used in the setting of suspected diverticulitis. A gentle, single-contrast study should be performed and terminated once findings of diverticulitis are discovered, with visualization of the entire colon deferred to a later date; air (double)-contrast studies are not indicated. Findings considered diagnostic of diverticulitis include demonstration of extravasated contrast material with or without the outlining of an abscess cavity, an intramural sinus tract, or a fistula.1,81 Extensive diverticulosis, spasm, mucosal thickening or spiking, or deformed sacs, although suggestive of diverticulitis, are not conclusive. An extraluminal mass compressing the colon is said to be the most common finding in severe diverticulitis,82 although this finding is not specific for this diagnosis. Obviously, in the absence of diverticula or associated findings, the diagnosis must be reconsidered. Contrast enema has been shown to have a sensitivity of 62% to 94% for detecting acute diverticulitis, with false-negative results in 2% to 15%.68,83
Computed Tomography
Because diverticulitis is mainly an extraluminal disease, luminal contrast studies may be inaccurate. Computed tomography (CT) (Fig. 117-4) has now replaced contrast enema as the diagnostic procedure of choice for acute diverticulitis and has the ability to image mural and extraluminal disease and to enable therapy with percutaneous drainage of abscesses. Abdominal and pelvic scanning ideally is performed with water-soluble contrast, given both orally and rectally, and with intravenous contrast when it is not contraindicated. CT criteria for diverticulitis include the presence of diverticula with pericolic infiltration of fatty tissue (often appearing as fat stranding), thickening of the colon wall, and formation of abscesses.