Chapter 22 Urologists care for patients with adrenal disorders principally from the surgical perspective. This chapter provides an overview of adrenal pathophysiology and addresses tactics used in urologic clinical practice to assess and manage surgical adrenal disease. 2. The right adrenal, described as triangular, lies posterior and lateral to the inferior vena cava (IVC) and contacts the liver anteriorly. 3. The smaller left gland tends to be semilunar and is intimately related to the upper pole of the left kidney. Anterior to the left adrenal lies the tail of the pancreas and the splenic artery. The diaphragm borders both adrenals posteriorly. Blood flow through the adrenals is substantial, given the endocrine function of the glands. Understanding adrenal vasculature is critical for safe retroperitoneal surgery. The venous drainage of the right gland differs from that of the left, and the surgeon must be aware of this fact. 1. No one dominant artery supplies either adrenal. 2. Small vessels enter the glands from three main sources: a. Inferior phrenic artery: superior adrenal arteries, majority of blood supply b. Aorta at the level of the superior mesenteric artery (SMA): middle adrenal arteries c. Renal artery: inferior adrenal arteries 3. Arteries rapidly branch, and as many as 60 separate vessels may penetrate the adrenal capsule in a stellate fashion. 1. Short vessel drains directly into the posterior aspect of the IVC. 2. If torn during dissection, can result in life-threatening bleeding and has been called “the vein of death”. 3. In some patients, joins with a right hepatic vein prior to entering the vena cava. 4. Accessory right adrenal vein(s) occasionally may be present. 1. The left adrenal vein is substantially longer than the right. 2. Drains directly into the left renal vein. 3. Usually joins with the inferior phrenic vein at some point in its course. 4. As on the right, accessory veins may be present. The adrenal gland, surrounded by a capsule, is composed of a yellowish cortex and a red-to-grey medulla. The cortex stems from the mesoderm, and the medulla originates from the neuroectoderm. a. Zona glomerulosa: mineralocorticoid production (outer zone) b. Zona fasciculata: glucocorticoid production (middle zone) c. Zona reticularis: androgen production (inner zone) 2. Medulla: This lies in the center of the gland beneath the zona reticularis. Rich in chromaffin cells (named so because precipitate chromium salts), the medulla produces catecholamines such as epinephrine, norepinephrine, and dopamine. At birth, the adrenal is of adult size. The gland rapidly begins to involute following delivery, losing tissue from a region known as the fetal cortex and weighing only 50% of its weight at birth when the child is 1 month of age. Each of the three zones of the cortex contains different enzymes along the three major synthetic pathways, resulting in three different classes of steroid hormones produced in each zone (Figure 22-1). The adrenal cortex and the adrenal medulla not only have distinct embryologic origins but also function as independent hormone-producing units. With cholesterol-derivative pregnenolone as substrate, numerous enzymes of the adrenal cortex catalyze synthesis of essential hormonal agents. Steroid hormones do not bind to receptors on cellular membranes but instead modulate gene transcription by uniting with their receptors within the cell and then binding directly to DNA as the receptor-hormone complex. 1. Zona glomerulosa is the site for daily production of up to 150 mg of aldosterone, the major mineralocorticoid in humans. Aldosterone is a pivotal player in the renin-angiotensin system and participates in control of the body’s fluid and electrolyte equilibrium. This hormone, through its action on the distal convoluted tubule of the kidney, promotes sodium (and therefore fluid) retention and potassium and proton excretion. Secretion of aldosterone is directly influenced by the body’s angiotensin II and potassium levels. It is important to note that the hypothalamic-pituitary-adrenal axis plays little role in aldosterone secretion. Indeed, the zona glomerulosa is the only region of the adrenal cortex that does not atrophy following loss of pituitary function. 2. Zona fasciculata is the site of glucocorticoid production and excretes up to 30 mg of cortisol each day. Unless pathology is present, cortisol secretion is under tight control of pituitary adrenocorticotropic hormone (ACTH) through the hypothalamic-pituitary-adrenal axis. Cortisol, essential for life, modulates numerous complex physiologic pathways that include metabolism, immunity, maintenance of intravascular volume, and regulation of blood pressure (BP). 3. Zona reticularis, the innermost zone of the adrenal cortex, is the source of adrenal androgens. The major sex steroid hormone of the adrenal is dehydroepiandrosterone (DHEA). In normal physiologic states, these hormones have little influence; however, excess production of these agents can lead to profound consequences (e.g., congenital adrenal hyperplasia [CAH]). Androstenedione and sulfated form of DHEA (DHEA-S) are also formed and, along with DHEA, act as weak androgens. The adrenal medulla is an integral part of the autonomic nervous system and the fight-or-flight response. Chromaffin cells, innervated by preganglionic sympathetic fibers, secrete norepinephrine, epinephrine, and dopamine. a. Catechol O-methyltransferase (COMT) b. Monoamine oxidase (MAO) 2. Catecholamine metabolites and small amounts of norepinephrine are excreted in the urine. The major metabolites include: b. Normetanephrine c. Vanillylmandelic acid (VMA) 1. Cushing syndrome: A condition of excess circulating glucocorticoid a. Signs and symptoms arise from chronic hypercortisolism. b. Causes of excess cortisol: Only a minority arise from the adrenal gland. 1) Primary adrenal hypercortisolism is a rare cause of Cushing syndrome. b) Bilateral micronodular hyperplasia is a rare cause of primary adrenal hypercortisolism. c) Bilateral ACTH-independent macronodular hyperplasia: Adrenals weigh up to 500 g each; this is extremely rare. 2) Nonadrenal causes of Cushing syndrome a) Iatrogenic steroid administration is a very common cause of Cushing syndrome. b) ACTH-producing pituitary tumors (Cushing disease) are responsible for up to 70% of cases of Cushing syndrome. Adrenal glands are hyperplastic (6 to 12 g) but less so than in nonpituitary ectopic ACTH cases. c) Ectopic ACTH is responsible for up to 15% of cases of Cushing syndrome. Half are caused by paraneoplastic action of small-cell lung cancer. Other malignancies such as carcinoid and pancreatic and thyroid tumors can also be culprits. Adrenal glands become extremely hyperplastic (12 to 30 g). d) Ectopic corticotropin-releasing hormone (CRH) is extremely rare. c. Comprehensive patient evaluation for Cushing syndrome is complex and is beyond the scope of this text. Diagnostic strategies as they relate to urologic practice, however, are discussed later in this chapter (see Evaluation of Adrenal Pathology in Urologic Practice). 2. Conn syndrome: Primary hyperaldosteronism a) Results from intravascular volume expansion b) Mean BPs of 180/110 mm Hg in patients with aldosterone-secreting adenomas and 160/100 mm Hg in those with idiopathic bilateral adrenal hyperplasia c) Renin levels are very low. d) Normotensive patients are extremely rare. 2) Hypokalemia a) Not present in all patients b) Balanced by the renal potassium-sparing effects of hypokalemia itself; can be exacerbated by sodium loading c) Can result in polyuria, polydipsia, and muscle weakness 3) Metabolic alkalosis is caused by potassium-induced H+ wasting. 4) Other metabolic abnormalities include mild hypernatremia (approximately 145 mEq/L) and hypomagnesemia. 5) Lack of edema is due to what has been called the “aldosterone-escape phenomenon.” Despite high mineralocorticoid levels, volume urinary fluid loss quickly equilibrates with intake, and volume expansion is only mild. 6) Other metabolic abnormalities include mild hypernatremia (approximately 145 mEq/L) and hypomagnesemia. 7) Increased cardiac risks: Patients with hyperaldosteronism have higher rates of cardiovascular events independent of hypertension and potassium levels. b. Causes of Conn syndrome a) Cause of up to 70% of cases of primary hyperaldosteronism b) Surgical disease ii) Treatment with preoperative spironolactone has been suggested. Long-term spironolactone or its counterpart eplerenone (lesser binding affinity to androgen and progesterone receptors) is used to treat poor surgical candidates. 2) Bilateral adrenal hyperplasia i) Responsible for 30% to 40% of Conn syndrome ii) Poor response to surgery—a medical disease: Aldosterone receptor blockade is employed. b) Glucocorticoid-suppressible aldosteronism i) Causes less than 1% of Conn syndrome ii) Autosomal dominant iii) Hyperaldosteronism is ACTH dependent and therefore responds to exogenous glucocorticoid administration. 3) Adrenal carcinoma is responsible for less than 1% of primary hyperaldosteronism. c. Evaluation of patients with hyperaldosteronism as it relates to urologic practice is addressed later in this chapter (see Evaluation of Adrenal Pathology in Urologic Practice). 3. Pheochromocytoma is a tumor of the adrenal medulla that generates, stores, and secretes catecholamines. 1) Classic symptom triad of headache, palpitations, and diaphoresis 2) Paroxysmal hypertension present in only approximately 50%, whereas other patients behave identically to those with essential hypertension. 3)
Disorders of the Adrenal Gland
Adrenal anatomy
Overview
Vasculature
Arterial supply
Venous drainage
Right adrenal vein
Left adrenal vein
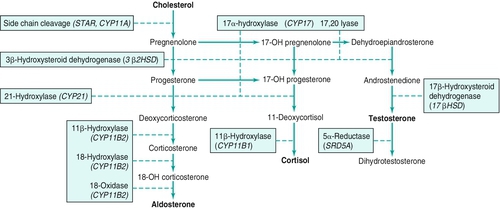
Histologic structure
Adrenal physiology
Adrenal cortex physiology
Adrenal medulla physiology
Adrenal disorders
Increased adrenal function
Cushing syndrome, conn syndrome, and pheochromocytoma
In cases of secondary hyperaldosteronism (usually due to renal artery stenosis or congestive heart failure [CHF]), renin levels are high.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


