Chapter 38 Diagnostic Pancreatography
Indications
Despite the reduction in use of ERP and ERCP, indications have remained essentially unchanged over the past decade. As for all endoscopic procedures, ERP is indicated only when the potential findings would alter the management of the patient’s condition in a meaningful way.1,2 Appropriate indications have been published by national societies and interest groups2 and revised for application in individual practice settings. Our standard indications for ERCP include investigation of symptoms strongly suspected to relate to the pancreas on the basis of associated abnormal laboratory or imaging tests, investigation of specific abnormalities on prior imaging or laboratory testing, and investigation of known pancreatic abnormalities for which intervention is needed or anticipated (Box 38.1). Diagnostic ERCP alone is not indicated to confirm findings clearly shown on other testing if therapy would not be changed by its performance. ERCP alone also is not indicated for investigation of isolated abdominal pain when other tests are normal. A National Institutes of Health consensus conference addressing the clinical applications of ERCP emphasized that it is indicated for investigation of isolated abdominal pain only when equipment and skills are available for concurrent therapy and for manometric investigation for potential sphincter of Oddi dysfunction.3
Preparation
Pancreatography may be performed alone or in concert with diagnostic or therapeutic endoscopic cholangiography (ERCP). Patient preparation, sedation, and positioning for pancreatography are the same as for endoscopic cholangiography. Recommendations for fasting intervals before sedation and intubation vary among centers.4 Most patients are asked to fast overnight; however, patients scheduled for afternoon procedures generally can be allowed a small clear liquid breakfast early in the day. Administration of antibiotics before the procedure is indicated in any patient with known or suspected parenchymal necrosis, duct obstruction, duct leak, or potential filling of poorly drained fluid collections or spaces such as peripancreatic fluid collections and pseudocysts.
Sedation for ERCP is usually accomplished by titrated parenteral administration of a narcotic and a benzodiazepine. Fentanyl is the narcotic of choice for most gastrointestinal (GI) endoscopy; however, compared with meperidine (Demerol), it has a shorter half-life and a greater stimulatory effect on the sphincter of Oddi.5,6 Fentanyl is less advantageous for performance of ERCP, and meperidine tends to be the narcotic of choice. Midazolam (Versed) is the usual benzodiazepine for all GI endoscopy, including performance of ERCP. Midazolam has been shown to reduce sphincter of Oddi pressures7,8 and is therefore not recommended during sphincter of Oddi manometry. Diazepam (Valium) does not influence the normotensive sphincter of Oddi, but little is known regarding its effects on the hypertensive sphincter. It is the preferred agent during manometric procedures.9 Propofol has become a popular agent for use during endoscopy by virtue of its extremely rapid induction and reversal and the deep sedation it provides. For ERCP, propofol is typically administered by an anesthesia specialist. Propofol does not seem to influence sphincter pressures at commonly used doses and can be used for all aspects of ERCP practice.10
Technique
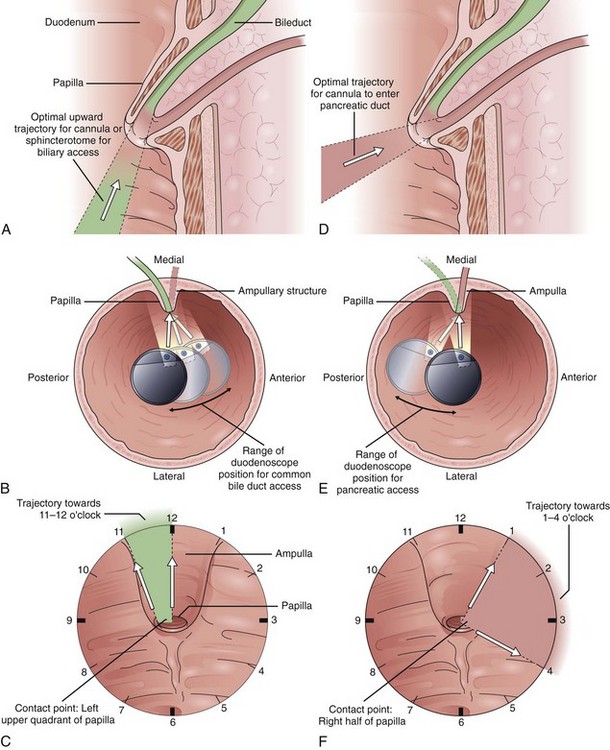
Endoscope positioning within the duodenum is crucial for efficient performance of cholangiography or pancreatography. For cholangiography, the optimal lens position is below the papilla looking upward or backward toward the proximal second portion of the duodenum. The cannula follows an upward and slightly posterior trajectory to access the bile duct located in the 10 o’clock to 12 o’clock position relative to the papillary os. In contrast, for pancreatography via the major papilla, the optimal lens position is relatively en face to the papilla with a slightly anterior directed view. The cannula is directed in a slightly forward and upward trajectory to access the pancreatic duct, which lies roughly between 1 o’clock and 3 o’clock relative to the papillary os (Fig. 38.1) (see Videos![]() ).11
).11
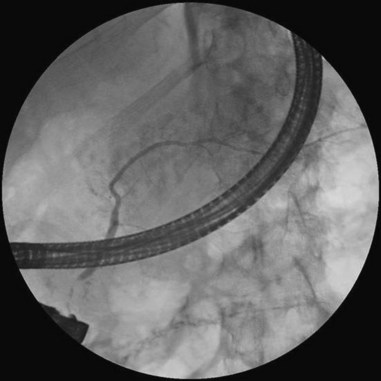
Cannulation of the major papilla is usually performed with the same equipment used in the biliary tree. Following apparent cannulation, fluoroscopically guided contrast agent injection should be more judicious and gradual than in the biliary tree. The normal pancreatic duct has a much smaller volume than the bile duct, and overfilling can occur after relatively small volumes of contrast agent have been injected; this may be recognized as acinarization, or filling of acini in a fluffy, more dense pattern throughout the distribution of the ducts (Fig. 38.2). Cannulation of normal side branches or of anomalous ductal systems can lead to overfilling of small segments almost immediately after injection is begun. Similarly, wire-guided cannulation must be more circumspect than in the bile duct because wire-induced perforation of a side branch can occur early, before contrast agent injection. When the appropriate position is confirmed within the desired system, more complete filling can proceed. Full-strength contrast agent is usually employed for pancreatography to optimize visualization of narrow ducts and fine detail.
In the setting of pancreatic fluid collections, for which formal drainage or surgery is not planned, caution should be employed to avoid excessive contamination of cysts that may not drain spontaneously. When difficult anatomy, pathology, or pancreas divisum prevents performance of pancreatography from the major papilla, it can be accomplished via the minor papilla approximately 90% of the time.12 The minor papilla is usually located 2 to 3 cm proximal and slightly anterior to the major papilla. It is usually less apparent than the major papilla and may not have an obvious opening. If identification of either the papilla or the os is difficult, secretin can be administered (0.2 mcg/kg body weight by intravenous injection over one minute) to stimulate the flow of pancreatic juice into the duodenum, which occurs within minutes of administration.13 Methylene blue can be sprayed on the duodenal wall to facilitate visualization of the focal source of drainage of clear pancreatic juice.14 Once identified, the minor papilla can be approached with a nearly en face view using either a semi–long scope position or an extremely short scope position with slightly posterior orientation. Cannulation usually follows a slightly posterior and horizontal to mildly cephalad path.
Complications of Pancreatography
The complications of diagnostic pancreatography are similar to the complications seen with therapeutic pancreatography and ERCP in general; a major exception is the rarity of perforation and bleeding. Complications related to sedation and intubation are similar. Pancreatitis is the predominant concern. The performance of pancreatography during ERCP is one of the most consistently positive risk factors identified in studies of procedural pancreatitis.15 Some studies note an even higher incidence with overfilling to the point of acinarization. Investigation of patients with past episodes of pancreatitis, particularly procedure-related pancreatitis, and investigation of patients with suspected sphincter of Oddi dysfunction are also risk factors for post-ERCP pancreatitis.
Numerous studies have investigated medications and interventions intended to reduce the incidence of post-ERCP pancreatitis. The details are beyond the scope of this chapter; however, on rigorous study, most interventions have not proven useful.15 Temporary prophylactic placement of a small-caliber pancreatic stent has been shown to reduce ERCP-related pancreatitis, or the severity of pancreatitis, in various patients,16 including patients in whom needle-knife sphincterotomy is used for access to the bile duct,15 patients in whom sphincterotomy is performed for treatment of sphincter of Oddi dysfunction,17 patients in whom sphincter dilation is performed for biliary stone removal,18 and patients with other high-risk indicators including “difficult cannulation.”19
Infection is a risk of pancreatography in predictable subgroups of patients, particularly patients with necrosis, duct leaks, and communicating cystic spaces such as pseudocysts. Although minimal data exist to guide the use of antibiotics in these settings, risk-to-benefit considerations suggest potential benefit of prophylactic antibiotics in the peri-ERCP period. In the past, pancreatic pseudocysts were considered a relative contraindication to ERCP, unless surgical drainage was planned within 24 hours. Experience has not supported this concern, however.20 Nonetheless, prudent practice might employ antibiotic prophylaxis and limited filling of chambers that would not effectively drain.
Normal Pancreatic Ductal Anatomy
Terminology for the linear extremes of the pancreatic duct is sometimes confusing because proximal and distal sometimes correlate with direction of flow (distal bile ducts are at the major papilla), but in the pancreas, proximal and distal usually refer to relative distance from a point of reference at the duodenal wall (proximal pancreatic duct relative to or near the major papilla). In line with this classic use, surgical terminology uses distal pancreatectomy to refer to resections beginning with the tail. To avoid confusion, especially as may occur among three specialties providing procedural, radiographic, and surgical interpretations, it is clarifying to speak of upstream segments or locations as being toward the tail relative to another location and downstream segments or locations as being toward the duodenum.21
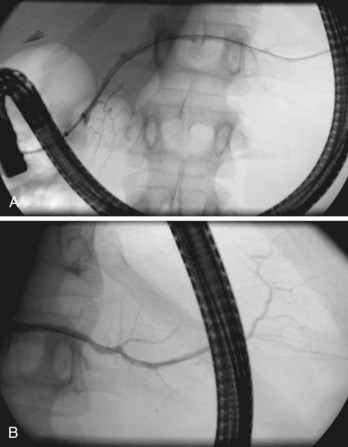
Globally, in the anteroposterior projection, the pancreatogram extends in an oblique fashion from the tail, located left of the spine at the T12 level, to the major papilla located right of the spine at the L2 level (Fig. 38.3). There is significant variation, however, in both the overall extent and the course of individual portions of the main duct. Within the head, the duct runs cephalad about 15 degrees from parallel to the spine; in the body, it runs horizontally or perpendicular to the spine; and in the tail, it usually rises further at a gentle angle but may even descend.22 The tail segment tends to be the most variable in course and shape. Occasionally, it is bifid, or split, left of the spine. In the anteroposterior projection, the retroperitoneal main pancreatic duct appears two-dimensional. However, oblique or lateral views show that it begins posteriorly in the tail, extends anteriorly around the spine, then extends back to a relatively posterior position at the genu and at the junction with the second portion of the duodenum. This excursion is highly variable and not useful for interpretation of displacement by adjacent space-occupying lesions.23
The main pancreatic duct is smooth with minor undulations and a general decline in caliber from the head to the tail. Focal nonpathologic indentations are sometimes noted at the genu, near the approximate junction with the accessory duct, and in the body, where it is in close proximity to the superior mesenteric vasculature.24 The former is well described but uncommon, and the latter is infrequently reported. Numerous studies have reported the length and caliber of the pancreatic duct as obtained from postmortem and endoscopic studies.25 Postmortem values tend to be slightly higher. With age, the duct caliber appears to increase slightly, while the length is stable. The length in normal glands averages about 16 to 17 cm, but it may range from 9 to 24 cm. When the main duct is less than 9 cm in length, obstruction should be suspected. The caliber of the pancreatic duct is most variable within the head of the gland, where the normal diameter is 3 to 4 mm but may range up to 6 mm, after correction for radiographic magnification. Accepted corrected diameters for the body and tail are 2 to 3 mm (up to 5 mm) and 1 to 2 mm (up to 3 mm).26
Visualization of side branches during pancreatography depends largely on technique and adequacy of contrast agent injection. Their visualization may be immaterial to the clinical question being addressed, or visualization may be crucial and of primary importance for resolution of clinical issues. Depending on the indication, lack of side-branch filling may not be indicative of a suboptimal or inadequate study. Side branches are highly variable and asymmetric in the pancreatic head but quite regular and symmetric, with alternating junctions along the main duct throughout the body and tail. A postmortem study reported a mean of 56 first-order branch ducts (range 52 to 66).27 Far fewer branch ducts are usually seen during even forceful pancreatography. A single large, inferiorly directed “uncinate” branch is seen in 55% to 62% of pancreatograms.24,28 The accessory pancreatic duct is shown in only 14% to 62% of endoscopic pancreatograms, even though postmortem studies can show its presence in 100% of autopsy specimens.25 It communicates with the main pancreatic duct in approximately 90% of specimens. Patency of the accessory duct and minor papilla together is highly variable. It is seen in more than 60% of general ERCP studies but in only 17% of studies in patients being evaluated for biliary pancreatitis, implying that when patent, the accessory duct serves to decompress the briefly obstructed main pancreatic duct.