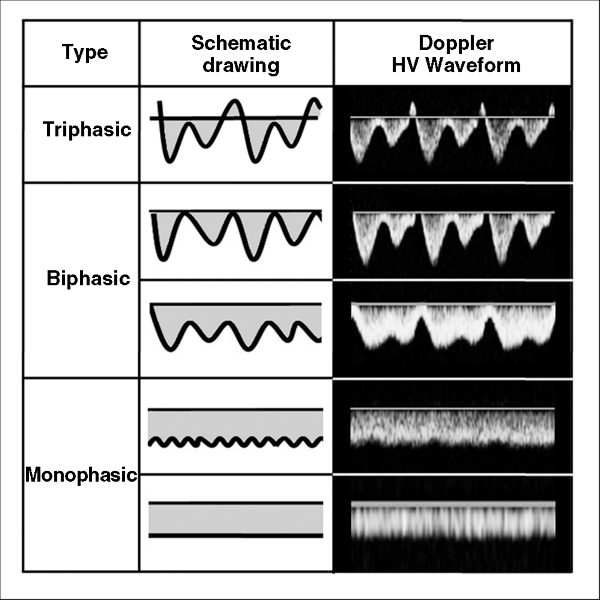
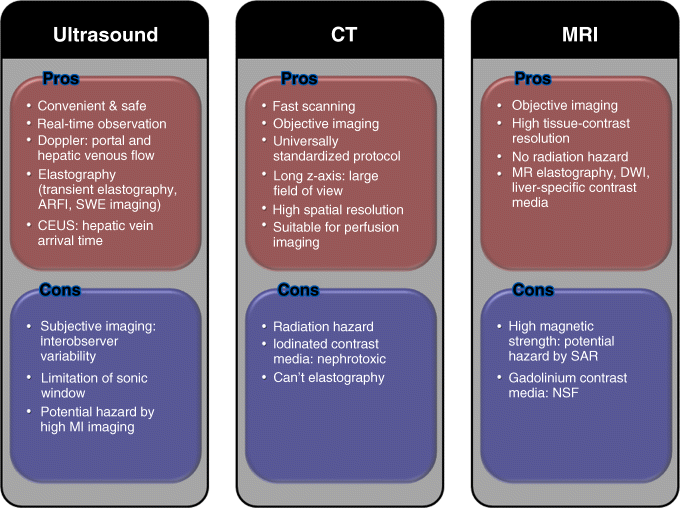
Chapter 3 Soon Koo Baik1, Moon Young Kim1, and Woo Kyoung Jeong2 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea 2Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea In chronic liver disease, diagnostic imaging has a significant role for patient management: diagnosis of hepatocellular carcinoma and progression to cirrhosis. As an antiviral treatment for viral hepatitis has been developed recently, the diagnosis of cirrhosis becomes more important for effective treatment. Basic diagnostic imaging modalities consist of ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI), and the many specific techniques derived from basic methods are developing for a convenient, non-invasive, and accurate diagnosis. Regardless of the underlying etiology, fibrosis is the main component of chronic liver damage that is directly related to disease severity and prognosis. Accurate estimation of the severity of fibrosis is essential for evaluating the disease state and prognosis, and is the first step toward the optimization of treatment and estimation of its response [1,2]. Liver biopsy is the gold standard for assessing fibrosis. However, standard clinical liver biopsy procedures have several limitations, including sampling errors, inter- and intraobserver variability, and invasiveness [3]. The ideal noninvasive test for diagnosing fibrosis would be simple and reproducible, readily available, less expensive than biopsy, and able to predict the full spectrum of fibrosis and to reflect any changes induced by therapy [4]. Many trials have been conducted with a view to finding this ideal test; however, no test has yet satisfied all of the aforementioned requirements. Moreover, recent advances in knowledge and treatment have led to proposals for more detailed histologic diagnosis of fibrosis, and these have made it even more difficult to find an ideal noninvasive substitute for liver biopsy sampling [5,6]. Various imaging modalities have been tried as methods for assessing liver fibrosis in chronic liver damage, including US, CT), and magnetic resonance (MR) based methods. This chapter reviews the role of various US tests used in the diagnosis and assessment of hepatic fibrosis, including grayscale US, Doppler US, contrast-enhanced US (CEUS), and elastography. Grayscale and Doppler US are noninvasive and relatively simple and inexpensive tests that are used to study and follow-up patients with chronic liver disease and cirrhosis. Various factors including the liver size, the bluntness of the liver edge, the coarseness of the liver parenchyma, the nodularity of the liver surface, portal vein (PV) velocity, and spleen size have been suggested as useful parameters for the US-based evaluation of chronic liver disease [7–14]. A previous study obtained a diagnostic accuracy ranging from 73% (Metavir score of ≥F2) to 84% (Metavir score of ≥F3) when using multiple grayscale US indices for fibrosis (i.e., liver length, liver surface nodularity, spleen length, and splenic vein respiratory variation) and one Doppler US index (i.e., PV velocity) [9]. Another study introduced a US scoring system using the surface pattern and the appearance of the internal echogenic bands, which relate to the irregularity of the liver texture, and demonstrated a significant correlation between US stages and the histologic staging of fibrosis [13]. Another study using the bluntness of the liver edge the irregularity of liver surface, and the coarseness of the parenchymal texture as indicators confirmed the usefulness of the US staging system [15]. However, although US can provide a qualitative assessment of the hepatic parenchyma composition, it is both subjective and operator dependent. Liver fibrosis and steatosis can have similar appearances on US and can be present at the same time in a “fatty-fibrotic pattern” [16–18]. In addition, some studies have shown that the sensitivity and specificity of US in demonstrating hepatic fibrosis are unacceptably low and that there is no correlation between US findings and the histologic stage of fibrosis on liver biopsy [19,20]. Regional hepatic and systemic hemodynamic changes are essential findings in liver fibrosis [21]. Therefore, Doppler US has been used to detect the hemodynamic changes that are known to be present from the pre-cirrhotic stages of hepatic fibrogenesis. Doppler US indices include the PV blood volume, mean or maximum PV velocity, portal blood flow, the congestion index of the PV, effective portal liver perfusion, and resistance indices of arteries in the liver and spleen [9,21–26]. Furthermore, pulsed wave Doppler can be applied to determine the changes in waveforms of the proper hepatic arteries, PV, and hepatic vein. While the normal flow pattern in the right hepatic vein is triphasic, patients with a biphasic or monophasic flow pattern more often have advanced fibrosis (Figure 3.1) [24,27–29]. Figure 3.1 Schematic drawings and US Doppler waveforms shows classification of hepatic vein Doppler waveforms. However, Doppler measurement is influenced by many patient-related factors such as respiration and timing of meals as well as observer variability and equipment differences. Furthermore, collateral pathways, hepatic steatosis, and inflammation further contribute to the measurement variability [30–32]. Taken together, grayscale and Doppler US are safe, inexpensive, and simple to use at the bedside or for outpatients, and combining multiple US indices can improve the diagnostic accuracy of cirrhosis in some conditions. CEUS imaging represents a new US modality for the assessment of chronic liver disease. CEUS involves the intravenous administration of minute, gas-filled microbubbles that strongly enhance the intensity of signals from the intravascular flow. The various commercially available contrast agents differ in their designs and kinetics, and can therefore yield different results; they cannot be used interchangeably [33–36]. Bolus injections of microbubble agents can be used for first-pass kinetics studies, and can be used to assess transit times. It has been shown that hepatic vein transit times shorten when the liver disease worsens [33,37,38]. In theory, shorter hepatic vein transit times in patients with diffuse liver disease occur mainly secondary to arteriovenous shunting and arterializations of capillary beds in the liver and, to a lesser degree, shunting in the pulmonary and gastrointestinal capillaries. In addition, the time–intensity curve differs significantly between the normal liver parenchyma and the cirrhotic liver, with the level of decrease being related to the degree of liver damage present [39]. Comparison of the signal intensity of a region of interest within the liver parenchyma has also revealed that the accumulation of microbubbles in the liver parenchyma is decreased in nonalcoholic steatohepatitis, but not in nonalcoholic fatty liver disease or chronic viral hepatitis [40]. In conclusion, CEUS could be a simple and noninvasive test for reliably screening cirrhosis based on contrast-agent transit or the parenchymal enhancement pattern. However, this method also has some limitations in that it requires the injection of a contrast agent, considerable operator skill, and access to the relevant technology. More intensive studies and validation are needed. The increase in liver stiffness associated with chronic liver disease is primarily because of the presence of fibrosis [41]. Tissue elastography, which was introduced in 1992, is used to visualize differences in the mechanical properties between tissues [42]. The most attractive advantage of tissue elastography is the ability to quantify the viscoelasticity of the tissue, which means that it can be used for measuring hepatic fibrosis in the liver. To date, two US elastography techniques have been used to measure liver stiffness: shear-wave-based elastography and real-time elastography. Shear-wave-based elastography includes transient elastography (TE), which is the most widely used, acoustic radiation force impulse (ARFI) imaging, and supersonic shear-wave elastography (SSWE). Shear-wave-based elastography involves using an ultrasonic beam to measure the propagation velocity of a shear wave through the soft tissue under investigation, with liver stiffness being displayed in kilopascals or centimeters per second. TE is the first commercialized elastography protocol developed to assess noninvasively the stiffness of deep soft tissues such as the liver. A mechanical vibrator generates a low-frequency elastic wave at 50 Hz to produce a shear stress in the target tissue at a distance of 4 cm, and then, as mentioned above, the velocity of the shear wave is measured using an ultrasound signal. TE has been validated extensively by numerous investigations targeted at patients with chronic liver disease and cirrhosis, and it is generally accepted that TE findings are strongly correlated with the stage of liver fibrosis. However, it does not provide a B-mode image (which is very helpful for targeting), only the right liver can be measured, and it has a higher measurement failure rate (5.5–6%), mainly because of limiting factors such as obesity and ascites [43]. ARFI imaging and SSWE use focused high-intensity, short-duration acoustic pulses instead of the mechanical vibration used in TE to produce a shear wave in the target tissue [44]. Like TE, the shear-wave velocity is observed by aiming repeated ultrasound beam pulses across the region of interest. SSWE is a new type of the shear-wave-based US elastography technique and uses multiple acoustic radiation force impulses [45]. It exhibits the remarkable feature of the viscoelastic properties in all areas within the region of interest being demonstrated on a color-coded look-up table. ARFI imaging and SSWE are expected to overcome the limitations of TE, such as measurement failures due to severe obesity, thick subcutaneous fat, and ascites. Moreover, they are able to display a grayscale US image on the background of the elastography, and so are more reliable and familiar to physicians who use conventional US. However, a high level of clinical experience and evidence are needed in order to apply either ARFI imaging or SSWE for the diagnosis of liver fibrosis. Real-time elastography is a method derived from the static elastography technique used for the measurement of breast tissue elasticity [46,47]. It differs technically from TE in that the echo signals obtained by conventional ultrasound probes before and when under slight compression are compared and analyzed. Tissue elasticity cannot be measured directly from reflected ultrasound echoes, and so the relative tissue elasticity is calculated and displayed as a color overlay on the conventional B-mode image. It is difficult to apply pressure to the liver using an ultrasound probe, so liver stiffness is calculated from the displacement of the tissue that results automatically from pressure applied by the heartbeat. Reflected US echoes are then used to compute the displacement and, thus, the strain distribution in the tissue. Many clinical studies have been conducted to test the effectiveness of TE for the diagnosis of hepatic fibrosis [48,49]. TE is useful as a screening test for cirrhosis, but is not recommended for diagnosing stages other than cirrhosis because the optimal cutoffs of liver stiffness have not been validated for individual stages of fibrosis. Relatively few clinical studies have been conducted on ARFI imaging and SSWE for comparison with TE, although some studies have shown that the performance and reliability of ARFI imaging and SSWE are comparable to those of TE [44,45,50]. Measurement of liver stiffness also has a major role in estimating the severity of portal hypertension in patients with cirrhosis. Increased portal pressure is the main factor determining the clinical course of cirrhosis: variceal formation, variceal bleeding, portal hypertensive enteropathy, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy are representative manifestations of decompensated cirrhosis. Measurement of the hepatic venous pressure gradient (HVPG) following hepatic venous catheterization is a surrogate marker of portal hypertension estimation, but this technique is somewhat invasive. Recent testing of the feasibility of liver stiffness measurement for detecting severe portal hypertension revealed a strong correlation with both HVPG and the presence of complications related to portal hypertension [51,52]. In conclusion, the development of various promising techniques for measuring liver stiffness have helped to overcome the limitations of conventional US, not only for evaluating hepatic fibrosis, but also for predicting the development of fatal complications related to chronic liver disease or portal hypertension, as well as the patient’s prognosis. As mentioned above regarding US, cross-sectional imaging studies such as CT and MRI are useful imaging modalities for the diagnosis of cirrhosis (Figure 3.2). These modalities are considered as standard methods for the diagnosis of hepatocellular carcinoma (HCC) on the background of chronic liver disease, including cirrhosis [53,54]. The radiologic features of advanced cirrhosis are normally obvious: surface nodularity, prominent fibrous septa, shrinkage of liver volume, and an enlarged portal venous system including varices and splenomegaly due to portal hypertension. However, it is difficult to diagnose the early stage of cirrhosis, and so various functional techniques using CT and MRI have been developed recently and described in many hepatology and radiology journals. This chapter describes the morphologic features of cirrhosis with various etiologies and the hemodynamic and functional imaging techniques for the early diagnosis of cirrhosis and its complications. Figure 3.2 Pros and cons of imaging modalities for diagnosis of cirrhosis. ARFI, acoustic radiation force impulse; CEUS, contrast-enhanced ultrasonography; CT, computed tomography; DWI, diffusion-weighted imaging; MR, magnetic resonance; MRI, magnetic resonance imaging; NSF, nephrogenic systemic fibrosis; SAR, specific absorption rate; SWE, shear-wave elastography. In the advanced stages of cirrhosis, the morphologic changes in the liver can be clearly demonstrated by both CT and MRI. Although the imaging features are highly specific for cirrhosis, the sensitivity is very low for early cirrhosis. The observed nodularity of the liver surface is caused by the presence of numerous regenerative nodules and fibrotic scars. Regenerative nodules are seldom evident on CT, but MRI reveals subtle changes such as regenerative nodules and strands of fibrosis. Regenerative nodules are composed of hepatocytes that regenerate in the fibrous scars following lobular hepatitis, and confluent necrosis of the hepatocytes [55]. Due to stepwise carcinogenesis from a regenerative nodule to a dysplastic nodule and then to HCC, cirrhotic nodules including regenerative and dysplastic nodules should be differentiated from HCC by using various MRI sequences and dynamic contrast enhancement. These cirrhotic nodules generally manifest as isoattenuating on CT, hypointense on T2-weighted MRI, and with no enhancement on arterial-phase CT and MRI, and are considered benign lesions [56]. Conversely, HCC appears with high signal intensity on T2-weighted MRI, as an early enhancement on arterial-phase CT and MRI because of its change of vascularity, and with high signal intensity on diffusion-weighted imaging (DWI) due to its high degree of cellularity [56,57]. Fibrotic scars are barely visible on conventional CT and MRI but, in patients with advanced cirrhosis, unenhanced MRI may reveal fibrotic septa as hypo- and hyperintense reticulations on T1- and T2-weighted MRI, respectively [58]. The use of either a gadolinium-based contrast agent or superparamagnetic iron oxide (SPIO) agent will result in hepatic fibrosis appearing as very clear contrast enhancement on MRI. Gadolinium-based contrast agents, except hepatocyte-specific agents such as gadoxetate disodium or gadobenate dimeglumine, equilibrate with the extracellular matrix of the liver in the delayed phase and accumulate in fibrotic liver tissue [59]. On the other hand, the SPIO agent is not taken up into fibrous septa, but rather into Kupffer cells in the hepatic sinusoid. As the signal intensity of the SPIO agent in the Kupffer cell is decreased on T2*-weighted gradient-recalled-echo sequence imaging, the fibrous septa are brighter than the regenerative nodules in which Kupffer cells are present [60]. A hybrid enhancement technique using sequentially both SPIO and gadolinium-based agents was also introduced to synergistically improve the diagnosis of hepatic fibrosis [61,62]. Cross-sectional imaging can be used for the diagnosis of cirrhosis; thus, several imaging signs related to morphologic changes of the liver have been investigated. A modified caudate : right-lobe ratio is one of these morphologic CT signs [63], defined by using the bifurcation of the right PV as a landmark, which is considered as a lateral boundary for the caudate lobe and a medial boundary for the right lobe. According to that study, the sensitivity and accuracy for diagnosing cirrhosis was about 72% and 74%, respectively, when using a modified caudate: right-lobe ratio cutoff of greater than 0.90. The expanded gallbladder fossa sign and the right posterior hepatic notch sign are other morphologic features visible on cross-sectional imaging in cirrhosis [64]. In the normal liver, the gallbladder fits into a fossa in the plane of the major interlobar fissure. In patients with cirrhosis, the fossa is widened because of atrophy of the right liver, and filled with fat tissue. The right posterior hepatic notch is a sharp pit on the right medial posterior surface of the liver that is usually noted in patients with cirrhosis. The use of these signs produces a very high specificity and positive predictive value for diagnosing cirrhosis, but usually a low sensitivity. In other words, the morphologic changes associated with cirrhosis that are visible on CT and MRI are usually observed in advanced cases only; CT and MRI are thus not suitable for the early diagnosis of cirrhosis. The progression to advanced disease will make atrophy of the right hepatic lobe and the left medial segment with hypertrophy of the left lateral segment and the caudate lobe more prominent, features that are easily demonstrated on conventional cross-section imaging modalities such as CT and MRI. These morphologic changes were previously explained by alterations of blood flow associated with the progress of cirrhosis. However, the morphologic changes differ depending upon the underlying etiology of the cirrhosis, although they overlap as the disease advances (Figure 3.3
Diagnostic Imaging Modalities
Ultrasonography-Based Approaches
Grayscale and Doppler US
Contrast-Enhanced Ultrasonography
Measurement of Liver Stiffness: Transient Elastography, Acoustic Radiation Force Impulse, Supersonic Shear-Wave Elastography, and Real-Time Elastography
CT- and MRI-Based Approaches
Morphologic Changes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








