Chapter 26 Diagnosis and Surveillance of Barrett’s Esophagus
Epidemiology
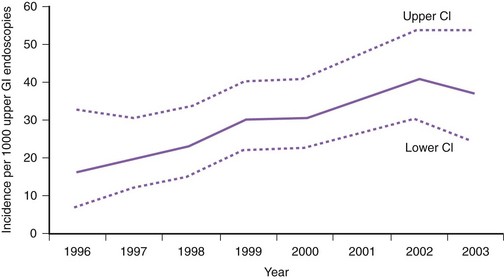
The incidence of Barrett’s esophagus has increased markedly since the 1970s. This increase was previously thought to be due to the increased use of diagnostic upper endoscopy combined with the change in the definition of Barrett’s esophagus to include shorter segments of columnar-lined epithelium.1 However, more recent data from the Netherlands suggest that the incidence of Barrett’s esophagus has increased from 14.3 per 100,000 person-years in 1997 to 23.1 per 100,000 person-years in 2002 in the general population independent of the number of upper endoscopies (Fig. 26.1).2
It is estimated that Barrett’s esophagus is found in approximately 5% to 15% of patients undergoing endoscopy for symptoms of GERD.3 A more recent study of a high-risk patient population (chronic GERD, white race, age >50 years) undergoing endoscopy for symptoms of GERD found Barrett’s esophagus in 13.2% of the subjects.3 Population-based studies suggest that the prevalence of Barrett’s esophagus is approximately 1.3% to 1.6%.4,5 Most of these patients in the general population have short-segment Barrett’s esophagus, and approximately 45% have no reflux symptoms.
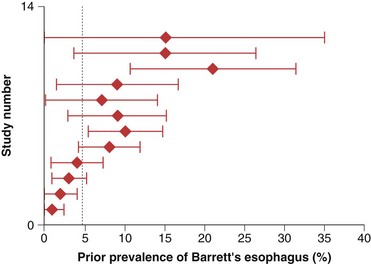
The prevalence of long-segment Barrett’s esophagus (≥3 cm of intestinal metaplasia) is approximately 5%, whereas the prevalence of short-segment Barrett’s esophagus (<3 cm of intestinal metaplasia) is approximately 6% to 12% in patients undergoing endoscopy in various settings.6–8 It is estimated that only 5% of patients undergoing resection for esophageal adenocarcinoma have a prior diagnosis of Barrett’s esophagus (Fig. 26.2).9
Barrett’s esophagus is predominantly a disease of middle-aged white men.10,11 However, approximately 25% of patients with Barrett’s esophagus are women or younger than 50 years of age.12,13 The prevalence of Barrett’s esophagus increases until a plateau is reached between the seventh and ninth decades.10,14 Various risk factors have been identified for the presence of Barrett’s esophagus, including frequent and long-standing reflux episodes, smoking, male gender, older age, and central obesity.15–19 Body mass index itself does not seem to be a risk factor for Barrett’s esophagus but rather the central obesity characteristic of male pattern obesity.17,18
Pathogenesis
Barrett’s esophagus is an acquired condition resulting from severe esophageal mucosal injury. It is unclear, however, why some patients with GERD develop Barrett’s esophagus whereas others do not. Animal studies suggest that the development of Barrett’s esophagus requires injury to the esophageal mucosa accompanied by an abnormal environment of epithelial repair.20 Epidemiologic data suggest that once injury occurs, Barrett’s esophagus develops to its full extent fairly rapidly with little subsequent change in length.10 The mechanism whereby injury triggers metaplasia and why this occurs in some but not all individuals is unknown. The cell of origin of columnar metaplasia is unclear. Candidates include dedifferentiation of squamous epithelium into columnar epithelium or stimulation of stem cells originating from the basal layer of the esophageal epithelium, esophageal submucosal glands, or bone marrow.21–23 The transcription factor CDX2, which can be induced by both acid and bile salts, seems to play a role in promoting the columnar epithelial differentiation pathway.24
Barrett’s esophagus is clearly associated with severe GERD. Compared with patients with erosive and nonerosive GERD without Barrett’s esophagus, patients with Barrett’s esophagus typically have greater esophageal acid exposure based on 24-hour pH monitoring.25,26 Part of the increase in acid exposure in patients with Barrett’s esophagus may be related to the almost uniform presence of a hiatal hernia, which is typically longer and associated with larger defects in the hiatus in patients with Barrett’s esophagus than in controls or patients with esophagitis alone.27,28 In addition, patients with Barrett’s esophagus have a lower basal lower esophageal sphincter pressure compared with GERD patients without Barrett’s esophagus.26 Reflux of duodenal contents is also increased in patients with Barrett’s esophagus compared with GERD patients without Barrett’s esophagus.29 Patients with short-segment Barrett’s esophagus tend to have pathophysiologic abnormalities intermediate to the abnormalities of patients with long-segment Barrett’s esophagus and normal controls.30,31 Esophageal pH monitoring studies suggest a correlation between the length of Barrett’s mucosa and the duration of esophageal acid exposure.31
Clinical Features
Patients with Barrett’s esophagus are difficult to distinguish clinically from patients with GERD uncomplicated by a columnar-lined esophagus.32 However, some observational studies suggest that features such as the development of reflux symptoms at an earlier age, increased duration of reflux symptoms, increased severity of nocturnal reflux symptoms, and increased complications of GERD (e.g., esophagitis, ulceration, stricture, bleeding) may distinguish patients with Barrett’s esophagus from GERD patients without Barrett’s esophagus.16 Similar clinical risk factors have been identified for esophageal adenocarcinoma.33 Identification of patients with Barrett’s esophagus may be hampered by the paradox that patients with Barrett’s esophagus have an impaired sensitivity to esophageal acid perfusion compared with patients with uncomplicated GERD.34 Many patients with Barrett’s esophagus are elderly, however, and this observation may be related to an age-related decrease in acid sensitivity.35 A subset of patients with Barrett’s esophagus may have an inherited predisposition; studies have reported families with multiple affected relatives over successive generations.36–38 These reports suggest an autosomal dominant pattern of inheritance in certain patients with Barrett’s esophagus.
Pathology
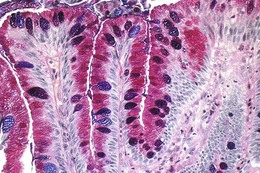
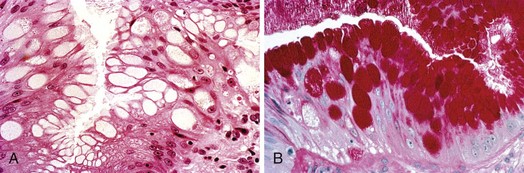
The columnar-lined esophagus is characterized by a mosaic of three different types of columnar epithelium above the lower esophageal sphincter zone: fundic-type epithelium, characterized by parietal and chief cells similar to the native gastric fundus; cardiac-type mucosa, characterized by mucous glands and no parietal cells; and specialized columnar epithelium, characterized by a villiform surface and alcian blue–staining intestinal-type goblet cells.39 At the present time, the diagnosis of Barrett’s esophagus is established if the squamocolumnar junction is displaced proximal to the EG junction and intestinal metaplasia, characterized by acid mucin–containing goblet cells is detected by biopsy (Fig. 26.3). The requirement of intestinal metaplasia for the diagnosis of Barrett’s esophagus has come under question more recently, however, as described later. In most cases, goblet cells are easily identified on routine hematoxylin and eosin preparations, and special stains such as alcian blue or periodic acid–Schiff (PAS) are unnecessary. However, alcian blue and PAS stain can help avoid overinterpretation of pseudogoblet cells characterized by distended surface foveolar-type cells that stain for PAS but do not contain alcian blue–positive acid mucins (Fig. 26.4).40
It is well recognized that pathologic interpretation of Barrett’s esophagus specimens is problematic in the community and in academic centers. In a community study, intestinal metaplasia without dysplasia was recognized correctly by only 35% of pathologists, and gastric metaplasia without intestinal metaplasia was identified as Barrett’s esophagus in 38% of the cases.41 Pathologic interpretation is also problematic for expert gastrointestinal (GI) pathologists; interobserver reproducibility is substantial at the ends of the spectrum of Barrett’s esophagus—negative for dysplasia and high-grade dysplasia and carcinoma but not especially good for low-grade dysplasia or indefinite for dysplasia.42 There are also problems with interobserver agreement among pathologists in distinguishing high-grade dysplasia from intramucosal cancer, even when evaluating esophagectomy specimens.43,44
Factors that contribute to some of the problems in pathologic interpretation include experience of the pathologist, quality of the slides, and size of the specimens.45 In an effort to improve pathologic interpretation, current practice guidelines now recommend endoscopic mucosal resection (EMR) of any nodularity in the Barrett’s segment before making final treatment decisions.46,47 More recent data support such an approach. Mino-Kenudson and colleagues45 found that interobserver agreement for Barrett’s esophagus–associated neoplasia on EMR specimens was higher than that for mucosal biopsy specimens, especially in distinguishing intramucosal cancer from submucosal cancer.
Differential Diagnosis
The diagnosis of Barrett’s esophagus has clear implications for patients. Patients are subject to surveillance endoscopy at regular intervals; worry about cancer risk, which they typically overestimate; face higher life insurance premiums; and are provided with conflicting information on how best to treat their condition.48,49 It is crucial to diagnose Barrett’s esophagus as accurately as possible given the downstream effects of such a diagnosis.
Barrett’s esophagus is defined as a metaplastic change in the lining of the tubular esophagus. Endoscopically, this metaplastic change is characterized by displacement of the squamocolumnar junction proximal to the EG junction defined by the proximal margin of gastric folds (Fig. 26.5). At the time of endoscopy, landmarks should be carefully identified, including the diaphragmatic pinch, the EG junction as best defined by the proximal margin of the gastric folds seen on partial insufflation of the esophagus, and level of the squamocolumnar junction. It is commonly accepted that the proximal margin of the gastric folds is the most useful landmark for the junction of the stomach and the esophagus.50 However, the precise junction of the esophagus and the stomach may be difficult to determine endoscopically because of the presence of a hiatal hernia, the presence of inflammation, and the dynamic nature of the EG junction, all of which may make targeting of biopsy specimens problematic.
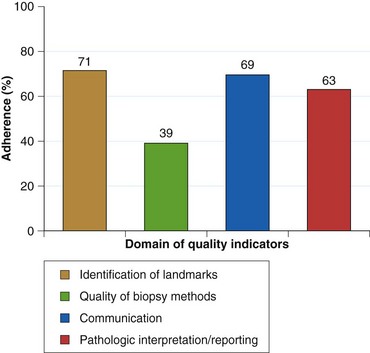
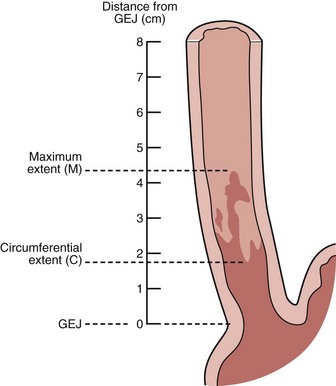
Endoscopists identify landmarks necessary for the diagnosis of the columnar-lined esophagus inconsistently (Fig. 26.6)51; this leads to inconsistencies in defining the length of the columnar-lined esophagus.52 The Prague classification scheme should help improve the description of the columnar-lined esophagus.53 This classification scheme describes the circumferential extent (C value) and maximum extent (M value) of columnar mucosa above the proximal margin of the gastric folds (Fig. 26.7). The Prague classification does not include columnar islands, however. Reliability coefficients for both criteria are excellent for segments greater than 1 cm in length. Recognition of less than 1 cm of columnar metaplasia even with this scoring system is still problematic, pointing out the difficulties in measuring such short segments.
If the squamocolumnar junction is above the level of the EG junction, as defined by the proximal margin of the gastric folds using partial insufflation, biopsy specimens should be obtained for confirmation of columnar metaplasia. There is ongoing debate regarding the presence of intestinal metaplasia for the diagnosis of Barrett’s esophagus.54 The professional societies of North America all require intestinal metaplasia for the diagnosis of Barrett’s esophagus, whereas the British Society of Gastroenterology and a global consensus group do not require the presence of intestinal metaplasia for the diagnosis.46,55–57 More recent evidence suggests that non–goblet cell columnar metaplasia shows DNA content abnormalities indicative of neoplastic risk similar to neoplasias encountered in intestinal metaplasia.58 The issue of intestinal metaplasia versus columnar metaplasia as a diagnostic criterion remains unsettled at the present time.
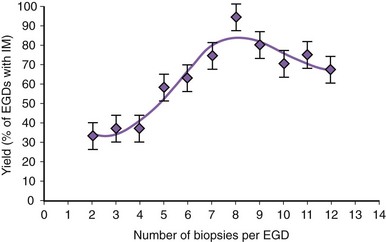
There is no agreement at the present time on the appropriate number of biopsy specimens to obtain to detect intestinal metaplasia. Detection of intestinal metaplasia seems to be related to many factors, including location of biopsies, length of columnar-lined segment, number of biopsy specimens obtained, male gender, and increasing age.59,60 Intestinal metaplasia is more commonly found in biopsy specimens obtained in the proximal portion of the columnar-lined esophagus where goblet cell density is also greater.61 More recent work suggests that the detection of intestinal metaplasia increases with increasing number of biopsy specimens per endoscopy: Four biopsy specimens had a yield of 34.7%, whereas eight biopsy specimens had a yield of 94% for intestinal metaplasia (Fig. 26.8).62 Taking more than eight biopsy specimens does not seem to enhance the yield of intestinal metaplasia.
It may be difficult to determine endoscopically where the esophagus ends and the stomach begins for the reasons outlined earlier. It is impossible to distinguish reliably columnar metaplasia of the distal esophagus from columnar metaplasia of the stomach. A biopsy specimen of the squamocolumnar junction should not be routinely obtained in clinical practice if it is at the level of the EG junction. Intestinal metaplasia may be seen in the cardia of normal individuals and in patients with chronic reflux disease. The prevalence of intestinal metaplasia at a normal-appearing EG junction varies from 5% to 36%.63 In contrast to Barrett’s esophagus, there is no clear gender predominance in patients with intestinal metaplasia of the EG junction and cardia because this condition is more common in older patients who are often infected with Helicobacter pylori and have evidence of gastritis or intestinal metaplasia or both elsewhere in the stomach.8,64 A subset of these patients may have GERD, however, and it is unclear if this condition is a sequela of aging, H. pylori infection, GERD, or some combination of these factors.
Short-segment Barrett’s esophagus is clearly associated with some risk of developing dysplasia and esophageal cancer, which is not substantially lower than the risk in patients with long-segment Barrett’s esophagus. Dysplasia and carcinoma have been reported in patients with intestinal metaplasia of the EG junction or cardia, but the magnitude of that risk seems to be less than the risk of short-segment Barrett’s esophagus.65 A reliable biomarker to distinguish between intestinal metaplasia of the cardia versus intestinal metaplasia of the esophagus would be beneficial. Techniques such as the Das-1 antibody and cytokeratin immunohistochemical staining patterns do not reliably distinguish between these two entities.66 However, some features such as mucosal and submucosal esophageal glands, squamous epithelium overlying columnar crypts with intestinal metaplasia, and hybrid glands characterized by intestinal metaplasia confined to the superficial aspect of cardia-type mucus glands are more often associated with the columnar-lined esophagus than intestinal metaplasia of the cardia.67 Precise targeting of biopsy specimens above the proximal margin of the gastric folds and communication of this information to pathologists are crucial from a quality perspective.
Barrett’s Esophagus and Esophageal Adenocarcinoma
Barrett’s esophagus is a clearly recognized risk factor for the development of esophageal adenocarcinoma compared with the general population.68 Studies show that the incidence of this cancer has increased by approximately sixfold between 1975 and 2001, a rate greater than that of any other cancer in the United States during that time.69 This increase has been accompanied by an increase in mortality rates from 2 to 15 deaths per 1 million during that same time period.69 Similar findings are occurring elsewhere in the Western world. However, the overall burden of esophageal adenocarcinoma remains relatively low. It was estimated that there would be 16,470 new cases of esophageal cancer (not all of which would be adenocarcinoma) in the United States in 2009.70
Despite the alarming increase in the incidence of esophageal adenocarcinoma, the precise incidence of adenocarcinoma in patients with Barrett’s esophagus is uncertain, with rates varying from approximately 1 in 52 to 1 in 694 years of follow-up.71 It is estimated that the risk of developing cancer in a patient with Barrett’s esophagus is approximately 0.5% to 0.7% annually with no clear evidence of geographic variation.71,72 Evolving epidemiologic data suggest that despite the alarming increase in the incidence of esophageal adenocarcinoma, most patients with Barrett’s esophagus never develop esophageal cancer and die of causes other than cancer.73–75 The survival of patients with Barrett’s esophagus is similar to survival of the general population.74
The reason for the increase in the incidence of esophageal adenocarcinoma is unknown. Barrett’s esophagus is clearly a risk factor for adenocarcinoma of the esophagus. Various epidemiologic factors have been identified that either increase or decrease the risk for the development of esophageal adenocarcinoma. The well-accepted risk factors for the development of esophageal adenocarcinoma include increasing age; male gender; white ethnicity; obesity, especially male pattern central obesity; and smoking.76–79 Protective factors include aspirin and nonsteroidal antiinflammatory drug (NSAID) ingestion and a diet high in fruits and vegetables.80–82 Factors of uncertain significance include family history, infection with H. pylori, alcohol consumption, antireflux therapy (surgical or pharmacologic), and dietary supplements.79
Cancer Biology
Compelling evidence exists for a dysplasia-carcinoma sequence in Barrett’s esophagus whereby nondysplastic columnar epithelium progresses to low-grade dysplasia, high-grade dysplasia, and finally to carcinoma. Foci of carcinoma typically appear adjacent to dysplasia.83 The time course for this progression is highly variable, and most patients never progress to dysplasia.
It is hypothesized that cancer develops in a subset of patients who have acquired genomic instability in Barrett’s epithelium.84 This genomic instability predisposes to the development of abnormal clones of cells that accumulate progressively more genetic errors, which include numerical and structural chromosomal rearrangements, gene mutations, loss of normal cell cycle control, and increased cell proliferation rates.85,86 Among the most frequently described molecular changes that precede the development of adenocarcinoma in Barrett’s esophagus are alterations in p53 (mutation, deletion, or loss of heterozygosity [LOH]) and p16 (mutation, deletion, promoter hypermethylation, or LOH) and aneuploidy.87–90 However, there is no clearly predictable sequence of genetic abnormalities that leads to the development of cancer. Upregulation of cyclooxygenase-2 (COX-2) expression also occurs in the metaplasia-dysplasia-carcinoma sequence.91,92 Increased COX-2 expression is associated with increased cellular proliferation and decreased apoptosis in vitro, and administration of selective COX-2 inhibitors can decrease cell growth and increase apoptosis in esophageal adenocarcinoma cell lines.93 This finding may have implications for chemoprevention strategies under investigation.
Screening and Surveillance Strategies for Barrett’s Esophagus
Esophageal adenocarcinoma is a lethal disease with a 5-year survival of approximately 12% to 14%.94,95 Survival depends on the stage, and early spread before the onset of symptoms is characteristic of this tumor. Early invasive cancer may be classified as intramucosal when neoplastic cells penetrate through the basement membrane to the lamina propria or muscularis mucosa and submucosal when neoplastic cells infiltrate into the submucosa. The prognosis for these two lesions is very different because the risk of lymph node metastasis is approximately 0% to 7% for intramucosal cancer but increases to 5% to 50% for submucosal cancer.96–99 Lymph node metastases are a clear prognostic factor for decreased survival.100 Approximately 95% of esophageal adenocarcinomas are diagnosed in patients without a prior diagnosis of Barrett’s esophagus.9 The best hope for improved survival of patients with esophageal adenocarcinoma is detection of cancer at an early and potentially curable stage.
Screening
One potential strategy to decrease the mortality rate of esophageal adenocarcinoma further is to identify more patients at risk, such as patients with Barrett’s esophagus. Population-based studies suggest that in patients with newly diagnosed esophageal adenocarcinoma, a prior endoscopy and diagnosis of Barrett’s esophagus was associated with both early stage cancer and improved survival.101 Only a few patients with esophageal adenocarcinoma have undergone prior endoscopy, however.102 Current professional society practice guidelines equivocate on screening patients with chronic GERD symptoms for Barrett’s esophagus.46,47,56 The 2009 American Cancer Society cancer screening guidelines does not include any recommendation for screening of either esophageal cancer or Barrett’s esophagus.103
Endoscopy with biopsy is still the only validated technique to diagnose Barrett’s esophagus. It has clear limitations as a screening tool, however, including cost, risk, and complexity. If screening with endoscopy and biopsy were applied to the estimated 20% of the population with regular GERD symptoms, the cost implications would be staggering.104 Unsedated upper endoscopy using small-caliber instruments still has the potential to change the economics of endoscopic screening because this technique may decrease sedation-related complications and costs. Unsedated small-caliber endoscopy detects Barrett’s esophagus and dysplasia with sensitivity comparable to conventional endoscopy.105 Although both procedures are well tolerated by patients, a major hurdle for unsedated endoscopy is patient resistance to undergoing a test without sedation. It is uncertain if endoscopy without sedation would meet with patient acceptance given the cultural preference for sedation in the United States. Otherwise, there are still no validated alternative techniques to screen for Barrett’s esophagus that overcome the cost and risks associated with conventional upper endoscopy.
There has been considerable interest in esophageal capsule endoscopy as a screening alternative to conventional upper endoscopy. Studies to date show a sensitivity of 60% to 79% and a specificity of 75% to 100% compared with conventional upper endoscopy.106–108 Modeling studies suggest that capsule endoscopy is not a cost-effective alternative to conventional endoscopy either.109 Adoption of esophageal capsule endoscopy as a screening alternative to upper endoscopy is unlikely in the near future.
After a normal initial upper endoscopy, some clinicians wonder if a repeat screening upper endoscopy should be undertaken in symptomatic GERD patients at a later date. Several studies have addressed this point with consistent results. In patients with nonerosive reflux disease at the index endoscopy, Barrett’s esophagus is rarely found if the repeat endoscopy is performed within 5 years.110,111 Barrett’s esophagus may be present in 9% to 12% of patients with erosive esophagitis at the time of index endoscopy, and higher grades of esophagitis are associated with a higher case finding rate of Barrett’s esophagus on repeat endoscopy.112,113 Screening for Barrett’s esophagus in GERD patients should take place only after initial therapy with a proton pump inhibitor (PPI). A negative endoscopy at baseline makes it highly unlikely to find Barrett’s esophagus if endoscopy is repeated.
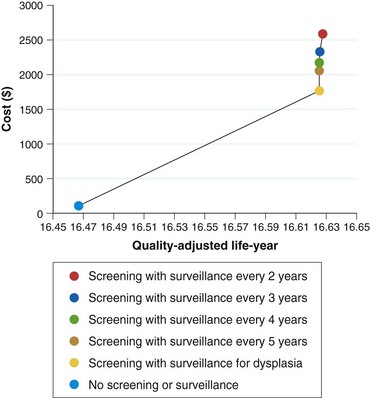
There are still no data from randomized controlled trials or observational studies to evaluate the strategy of screening. A decision analysis model by Inadomi and colleagues114 examined screening of 50-year-old white men with chronic GERD symptoms for Barrett’s esophagus and found that one-time screening is probably cost-effective if subsequent surveillance is limited to patients with dysplasia on initial examination (Fig. 26.9). This strategy would result in a cost of $10,440 per quality-adjusted life year saved compared with a strategy of no screening or surveillance. Other modeling studies support screening in patients with chronic GERD symptoms as well but only if the following conditions are met: patients at high risk for Barrett’s esophagus, high-grade dysplasia, or adenocarcinoma; high sensitivity and specificity of endoscopy with biopsy; and little or no reduction in quality of life with esophagectomy.115,116 Any variation of these ideal conditions quickly made this strategy cost-ineffective.
There is clearly a need to develop either a better profile of patients at high risk for Barrett’s esophagus and high-grade dysplasia or a far less expensive tool to provide mass population screening. A simple questionnaire and nomogram has been described in an effort to predict Barrett’s esophagus in patients with GERD symptoms.117 The sensitivity of this questionnaire for predicting Barrett’s esophagus was 77% with a specificity of only 63%. Although clearly cost saving, this questionnaire would miss patients with Barrett’s esophagus with GERD symptoms and not account for individuals without any symptoms of GERD.
Surveillance
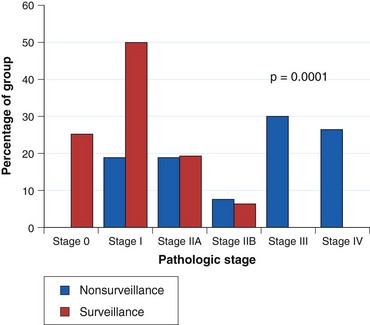
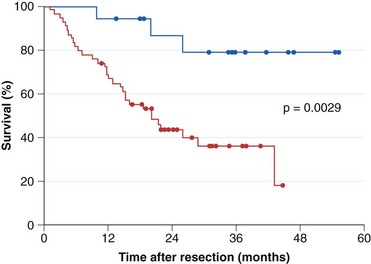
Current practice guidelines recommend endoscopic surveillance of patients with Barrett’s esophagus in an attempt to detect cancer at an early and potentially curable stage.46,47,55,56 Numerous observational studies suggest that patients with Barrett’s esophagus in whom adenocarcinoma was detected in a surveillance program have their cancers detected at an earlier stage (Fig. 26.10), with markedly improved 5-year survival compared with similar patients not undergoing routine endoscopic surveillance (Fig. 26.11).102,118–121 Nodal involvement is far less likely in patients undergoing surveillance compared with patients not undergoing surveillance.120 Because survival in esophageal cancer is stage-dependent, these studies suggest that survival may be enhanced by endoscopic surveillance. Several decision-analysis models support the concept of endoscopic surveillance.114,122,123 The model of Provenzale and coworkers122 suggests that surveillance every 5 years is the most effective strategy to increase length and quality of life, whereas the model of Inadomi and colleagues114 suggests that surveillance should be limited only to individuals with dysplasia at the time of initial endoscopy.
Other authors argue that because most patients with Barrett’s esophagus do not die of esophageal cancer, the benefit for surveillance remains uncertain, and endoscopic surveillance is not warranted until substantiated by prospective studies.124 Design flaws such as selection bias, healthy volunteer bias, lead time bias, and length time bias are inherent in the observational studies that support endoscopic surveillance. The resources encumbered by vigorous endoscopic surveillance are considerable. Despite the concern regarding the esophageal cancer “epidemic,” the overall burden of disease is limited in the Western world compared with other malignancies such as colon cancer. A randomized controlled trial of surveillance versus no surveillance in Barrett’s esophagus has not been performed and is not likely to be performed in the future.
Candidates for Endoscopic Surveillance
Patients with documented Barrett’s esophagus are candidates for surveillance. Before entering into a surveillance program, patients should be advised about risks and benefits, including the limitations of surveillance endoscopy and the importance of adhering to appropriate surveillance intervals.47,56,125 Other considerations include age, likelihood of survival over the next 5 years, and ability to tolerate either endoscopic or surgical interventions for early esophageal adenocarcinoma.
Surveillance Techniques
The aim of surveillance is to detect dysplasia. The description of dysplasia should use a standard five-tier system: (1) negative for dysplasia, (2) indefinite for dysplasia, (3) low-grade dysplasia, (4) high-grade dysplasia, and (5) carcinoma.42 Active inflammation makes it more difficult to distinguish dysplasia from reparative changes. It is essential that surveillance endoscopy is performed only after any active inflammation related to GERD is controlled with antisecretory therapy. The presence of ongoing erosive esophagitis is a contraindication to performing surveillance biopsies.
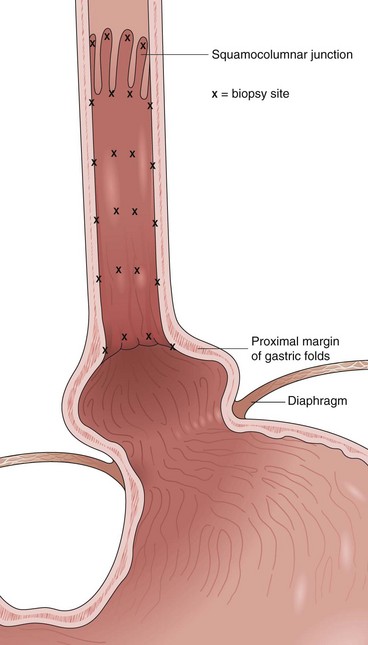
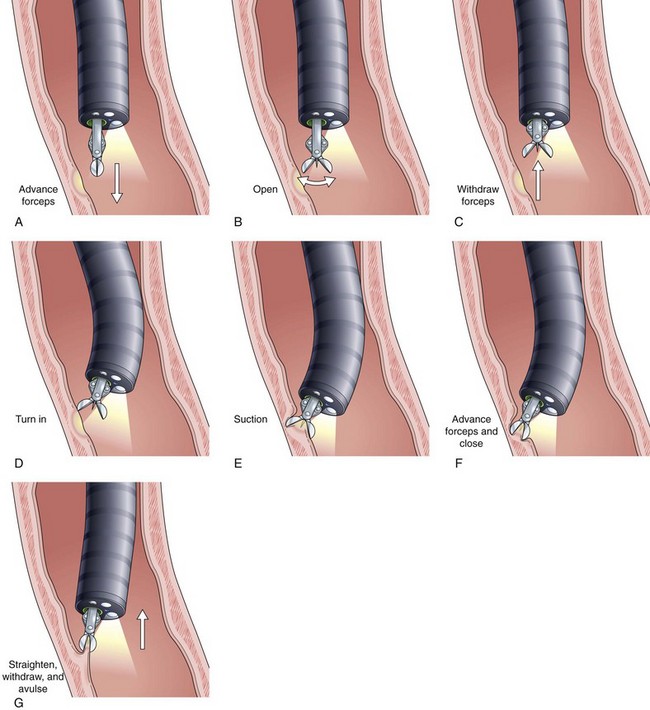
Current guidelines suggest obtaining systematic four-quadrant biopsy specimens at 2-cm intervals along the entire length of the Barrett’s segment after inflammation related to GERD is controlled with antisecretory therapy (Fig. 26.12).46,47,55,56 A systematic biopsy protocol clearly detects more dysplasia and early cancer compared with ad hoc random biopsies.126,127 Extensive biopsies of subtle mucosal abnormalities, no matter how trivial, such as ulceration, erosion, plaque, nodule, stricture, or other luminal irregularity in the Barrett’s segment, should also be performed because there is an association of such lesions with underlying cancer.128 Current guidelines also recommend, however, that patients with mucosal abnormalities, especially in the setting of high-grade dysplasia, should undergo EMR.46,47 Studies suggest that EMR changes the diagnosis in approximately 50% of patients, given the larger tissue sample available for review by the pathologist.129 The “turn and suction” technique (Fig. 26.13) allows acquisition of biopsy specimens that are significantly larger than the specimens obtained by the traditional techniques of advancing an open biopsy forceps into the lumen and then closing it to obtain the biopsy sample.130 The safety of systematic endoscopic biopsy protocols has been shown.131
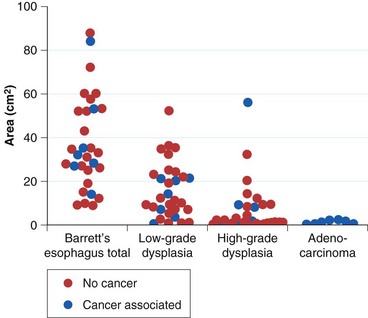
The rationale for such a comprehensive biopsy program comes from observations that high-grade dysplasia and early carcinoma in Barrett’s esophagus often occur in the absence of endoscopic abnormalities and from the focal nature of dysplasia. Systematic esophagectomy mapping studies show just how focal dysplasia and superficial cancer may be. In 30 esophagectomy specimens from patients undergoing surgery for either high-grade dysplasia or early invasive adenocarcinoma with no endoscopic evidence of cancer, the median surface area of total Barrett’s esophagus was found to be 32 cm2; low-grade dysplasia, 13 cm2; high-grade dysplasia, 1.3 cm2; and adenocarcinoma, 1.1 cm2 (Fig. 26.14).132 The three smallest cancers had surface areas of 0.02 cm2, 0.3 cm2, and 0.4 cm2.
Because of the focal nature of dysplasia and cancer, some experts recommend that endoscopic surveillance should use a large particle (jumbo) forceps to obtain biopsy specimens.133 However, other authors have found that jumbo forceps biopsies still miss cancer in patients with high-grade dysplasia if the biopsies are performed at both 1-cm and 2-cm intervals.134 This technique requires passage of a therapeutic endoscope, and the generalizability of this technique to clinical practice is problematic. Large-capacity forceps are available for passage through standard diameter endoscopes, which should render this issue less important than in the past. The extensive use of EMR has changed biopsy sampling considerably. Current guidelines suggest that available evidence does not support the routine use of the jumbo biopsy forceps.47
Surveillance Intervals
Surveillance intervals, determined by the presence and grade of dysplasia, are based on the limited understanding of the biology of esophageal adenocarcinoma. The most recently published recommendations from the American College of Gastroenterology are shown in Table 26.1. These intervals are arbitrary, however; they have never been subject to a clinical trial and likely never will be. Guidelines from various professional societies are not in agreement on surveillance intervals or techniques. The American College of Gastroenterology46 and the American Society for Gastrointestinal Endoscopy55 both recommend surveillance every 3 years as adequate in patients without dysplasia after two negative examinations. The American Gastroenterological Association47 recommends extending the surveillance interval up to 5 years, whereas the British Society of Gastroenterology56 recommends continued surveillance at 2-year intervals in this setting.
Table 26.1 2008 American College of Gastroenterology Practice Guidelines for Endoscopic Surveillance of Barrett’s Esophagus
| Dysplasia Grade | Interval |
|---|---|
| None | Every 3 yr after two endoscopies are negative within 1 yr |
| Low-grade | Expert GI pathologist confirmation |
| Repeat endoscopy within 6 mo to ensure no higher grade of dysplasia | |
| Every year until no dysplasia on two consecutive endoscopies | |
| High-grade | Expert GI pathologist confirmation |
| If Barrett’s segment flat—redo endoscopy and biopsies within 3 mo | |
| If mucosal abnormality—endoscopic mucosal resection | |
| Counsel patient with options | |
| Intensive surveillance | |
| Endoscopic therapy | |
| Esophagectomy |
GI, gastrointestinal.
Adapted from Wang KK, Sampliner RE: Updated guidelines 2008 for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 103:788–797, 2008.
If low-grade dysplasia is found, the diagnosis should first be confirmed by an expert GI pathologist owing to the marked interobserver variability in interpretation of these biopsy specimens. Data suggest that if there is a consensus diagnosis by two or three expert GI pathologists, the risk of progression is greater than if there is no such agreement.135 These patients should receive aggressive antisecretory therapy for reflux disease with a PPI to decrease the chances of regeneration that make pathologic interpretation of this category so difficult. A repeat endoscopy should be performed within 6 months of the initial diagnosis. If low-grade dysplasia is confirmed, annual surveillance is recommended when low-grade dysplasia is present until two examinations in a row are negative.46 There is no agreement on the biopsy protocol to use, although a protocol of four-quadrant biopsy specimens at 1-cm intervals as would be used for high-grade dysplasia makes sense. EMR should be performed if any mucosal abnormality is present in these patients.
If high-grade dysplasia is found, the diagnosis should first be confirmed by an experienced GI pathologist as well. If the segment is flat and without any mucosal abnormalities, the endoscopic biopsy protocol should be repeated within 3 months to exclude an unsuspected carcinoma using careful inspection with high-quality white light endoscopy.46 It is still unclear how much enhanced imaging techniques add to careful inspection with high-resolution or high-definition white light endoscopy (see later). The presence of any mucosal abnormality warrants EMR in an effort to maximize staging accuracy.
If high-grade dysplasia is confirmed, there is no consensus on the appropriate management of these patients. Options include continued surveillance, endoscopic therapy, or esophagectomy. Although continued surveillance has been compared with endoscopic approaches in randomized controlled trials, esophagectomy has not been compared with endoscopic ablative therapy in any randomized controlled trials.136 Observational studies suggest, however, that survival and cancer-free survival are comparable in patients treated either surgically or endoscopically for high-grade dysplasia.137 If continued surveillance is chosen, one proposed option is surveillance at 3-month intervals for 1 year.138 If there is no high-grade dysplasia on two consecutive endoscopies for the first year, endoscopy frequency is lengthened to every 6 months for the second year and then to annually thereafter as long as high-grade dysplasia is not encountered again. If high-grade dysplasia persists, continued short-interval endoscopy is warranted.
The extent of high-grade dysplasia is thought by some authors to be a risk factor for the subsequent development of adenocarcinoma.139 However, there are currently no uniform criteria for defining the extent of high-grade dysplasia, and there are conflicting data on the clinical significance of extent of high-grade dysplasia in biopsy specimens and risk for unsuspected carcinoma.139,140 Mucosal abnormalities in patients with multifocal high-grade dysplasia may also be a risk factor for adenocarcinoma.141,142 High-grade dysplasia remains a worrisome lesion, although progression to carcinoma may take many years and is not inevitable. The ultimate approach to a patient with high-grade dysplasia should consider factors such as available surgical and endoscopic expertise; age of the patient; length of Barrett’s epithelium that would require biopsy to eliminate sampling error; compliance with endoscopic surveillance; future need for multiple surveillance endoscopies; and suspicious lesions such as plaques, nodules, and strictures.