Chapter 25 Diagnosis and Staging of Esophageal Carcinoma
Introduction
The incidence of esophageal carcinoma is increasing in many countries, and the disease remains highly lethal because most patients present with advanced disease. Squamous cell carcinoma (SCC) and adenocarcinoma are the most common subtypes, whereas verrucous carcinoma (a variant of SCC) and small cell carcinoma occur rarely. Nonepithelial tumors of the esophagus are discussed in Chapter 29. Diagnosis of early SCC and its precursors is discussed in Chapter 30. Diagnosis and surveillance of Barrett’s esophagus is described in Chapter 26. The use of emerging endoscopic technologies in the diagnosis and assessment of early esophageal neoplasia is discussed in Chapter 27.
Epidemiology
Carcinoma of the esophagus ranked as the eighth most common carcinoma worldwide in 1990 and accounts for 7% of gastrointestinal (GI) malignancies.1 Most patients in the Western world present after the age of 65 years. Approximately 13,900 people in the United States are affected annually with an overall age-adjusted incidence of 4.5 per 100,000, ranging from 2.1 per 100,000 in women to 7.7 per 100,000 in men.2,3 Overall 5-year survival has improved in recent years to 14%, which is still low. There are significant epidemiologic differences between the two main carcinoma subtypes, and these are considered separately.
Squamous Cell Carcinoma
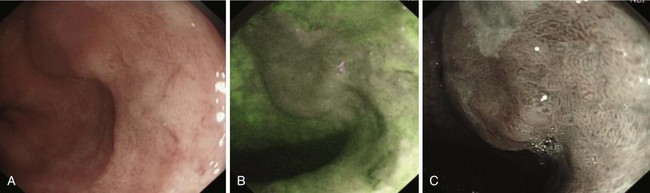
SCC can arise anywhere in the esophagus with an approximately equal distribution throughout (Fig. 25.1). Globally, SCC is more common than adenocarcinoma with an overall incidence of 2.5 to 5.0 per 100,000 for men and 1.5 to 2.5 per 100,000 for women.4 There are, however, striking geographic variations in incidence. High-risk areas include the Transkei region of South Africa and the so-called “Asian esophageal cancer belt” comprising eastern Turkey, India, northern Iran, and northern China, where the incidence is greater than 100 per 100,000 population.5 In the United States, African American men have the highest incidence of any ethnic group (16.8 per 100,000), and men are generally affected two to three times more often than women,6 although the incidence is similar in the two groups in high-risk areas of the world. The disease is most prevalent among lower socioeconomic groups.
Variations in incidence have been attributed to numerous etiologic factors, including environmental exposure, dietary habits, infection, radiation exposure, and associated high-risk conditions. The most important risk factors for SCC (in the Western world) are tobacco and alcohol use,7,8 the risk being greatest in individuals who both smoke and drink. Many carcinogens, including nitrosamines and polycyclic hydrocarbons, exist in both tobacco smoke and alcohol, and the risk may be compounded in alcohol abusers by concomitant nutritional and immunologic deficiencies.5
Numerous high-risk conditions have been described. Achalasia may predispose to SCC in association with chronic stasis of food and debris in the dilated esophagus, with an incidence of SCC of up to 9% after 15 to 20 years.5 Lye strictures of the esophagus are associated with a 1000-fold increased risk of SCC compared with controls, with patients often presenting 40 to 50 years after lye ingestion. Up to 16% of patients with Plummer-Vinson syndrome (iron deficiency anemia, dysphagia, and postcricoid webs in elderly women) may develop pharyngeal or esophageal carcinoma.9 Long-standing celiac disease is rarely associated with esophageal and pharyngeal SCC in addition to enteropathy-associated T-cell lymphoma and adenocarcinoma.10 Tylosis is a rare autosomal dominant disease consisting of palmar and plantar hyperkeratosis along with thickening and fissuring of the skin. Up to 95% of patients develop esophageal SCC by age 65 years.11 Patients with SCC of the head and neck have been reported to develop synchronous esophageal carcinoma in up to 8% of cases12 with tobacco and alcohol as common risk factors for both.
Adenocarcinoma
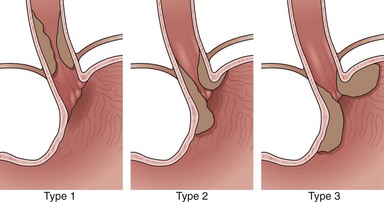
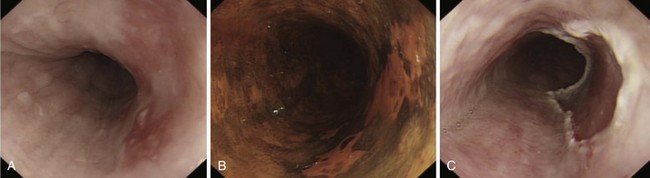
Adenocarcinomas arise in the distal esophagus and at the esophagogastric (EG) junction (Fig. 25.2). Tumors at the EG junction and gastric cardia were previously considered as primary gastric carcinomas invading the distal esophagus. These tumors have been reclassified (Fig. 25.3) as type 1 when they arise from the distal esophagus, type 2 when the origin is the gastric cardia, and type 3 when they are subcardial.13 Although this classification should allow more accurate characterization of tumors at the EG junction into primary esophageal or primary gastric types, changes in terminology and classification over time must be borne in mind when interpreting data from relevant studies.
Current evidence suggests that type 1 (and possibly type 2) carcinomas of the EG junction have a similar clinical course to distal esophageal tumors and should be staged as for esophageal carcinoma.14 Epidemiologic differences between esophageal adenocarcinoma and type 3 adenocarcinoma of the EG junction suggest that these diseases are separate entities,15 and type 3 tumors are usually staged according to criteria for gastric cancer.
In contrast to SCC, the incidence of esophageal adenocarcinoma has been increasing in many parts of the developed world since the 1970s, a consistent finding across the United States, Europe, and Australasia.16–18 The most rapid increases have been reported in the United States at 10% per year,19 especially in white men, and adenocarcinoma is now more common than SCC.16 Current incidence in the developed world is approximately 4.0 per 100,000 population, varying from less than 1.0 in Eastern Europe to 5.0 to 8.7 per 100,000 in Great Britain.3,4 Similar trends have not been observed to date in Asian populations.20
The predominant risk factors for adenocarcinoma are gastroesophageal reflux (GER) and Barrett’s esophagus. In contrast to SCC, lower socioeconomic status and tobacco and alcohol use are not strongly associated with increased incidence,21 although smoking has been found to be a risk factor in some studies.22,23 Chronic GER predisposes to Barrett’s esophagus24 but was also associated independently with the development of adenocarcinoma in a large study of Swedish patients.25 In this population, the development of adenocarcinoma correlated with duration, frequency, and severity of GER. Obesity has also been implicated as an independent risk factor in the development of adenocarcinoma, perhaps because of an influence on GER. A meta-analysis of eight studies reported an odds ratio for esophageal adenocarcinoma of 1.52 (95% confidence interval 1.147 to 2.009) in patients with a body mass index of 25 to 30 kg/m2, increasing to 2.78 (95% confidence interval 1.850 to 4.164) in patients whose BMI was greater than 30 kg/m2.26 There are wide variations in the reported relative risk of cancer development in Barrett’s esophagus, but it is estimated to be approximately 0.5% per year (i.e., 1 in 200 patient-years).24,27 There is a weak correlation between length of the Barrett’s segment and malignant potential.28
Clinical Features
The esophagus lacks a serosal covering, and early tumor growth causes asymptomatic dilation of the smooth muscle. In most patients, dysphagia results only when the lumen has narrowed to 50% to 75% of its normal circumference. By this time, local or nodal spread has often occurred, and the tumor is incurable. Cancers can manifest early with dysphagia, but this is uncommon. Dysphagia may be present for several months before medical advice is sought, and subjective localization of the site of obstruction is a poor indicator of the actual site of disease in the esophagus.29 Symptoms are commonly gradual in onset, with progressive difficulty swallowing solids and then liquids. In rare cases, EG junction tumors infiltrating the submucosa affect motility and result in “pseudoachalasia” with no obvious endoscopic mucosal abnormality and clinical features similar to achalasia.
Anorexia and weight loss, which often precede the onset of dysphagia, are common, and odynophagia may occur with ulcerated tumors. Persistent retrosternal pain, unrelated to swallowing, and back pain are sinister symptoms suggesting mediastinal invasion.29 Cough worsened by swallowing or recurrent pneumonia suggests esophagobronchial fistulization. This complication occurs in 5% to 10% of patients and is associated with poor outcome resulting in a median survival time of 1.5 to 4 months.30 Hematemesis is uncommon.31 Exsanguination resulting from aortoesophageal fistula is rare. Tumor involvement of the left recurrent laryngeal nerve results in hoarseness.
Esophageal carcinoma progresses through direct extension, lymphatic spread, or hematogenous metastasis or a combination of these. Lack of a serosal covering facilitates direct extension and invasion of structures in the neck or chest. Early lymphatic spread via rich interconnecting lymphatic networks in the esophageal submucosa is common. Tumors at any site may involve nodes in the neck or the mediastinum, although proximal lesions metastasize more commonly to cervical nodes, and distal lesions metastasize to abdominal nodes.32 Depth of invasion is a major determinant of lymph node metastasis. Disease confined to the mucosa is associated with nodal involvement in less than 5% of cases,33 whereas penetration into the submucosa is associated with a 15% to 50% risk of metastases.34,35 Hematogenous spread occurs to the lungs, liver, adrenals, kidneys, pancreas, peritoneum, bones, and brain.
Diagnosis
Esophagogastroduodenoscopy
Indications and Contraindications
Flexible videoendoscopy with biopsy is the “gold standard” investigation for the diagnosis of esophageal carcinoma (see Figs. 25.1 and 25.2). Endoscopy is more sensitive and specific than double-contrast barium for the diagnosis of upper GI cancer,36 and when biopsy and cytology are combined, the accuracy of endoscopy for diagnosis approaches 100%.37 Rigid esophagoscopy is rarely necessary and is no longer recommended on the grounds of safety and cost-effectiveness.38 In rare patients with pseudoachalasia and repeated negative mucosal biopsy results, endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA) biopsy from within the esophageal wall may provide supportive evidence for malignancy and a tissue diagnosis.39
Equipment
Good-quality videoendoscopes are essential. For assessment of dysplasia or early neoplasia, high-resolution instruments with magnification and instruments with enhanced imaging techniques (e.g., autofluorescence, narrow band imaging, or confocal endomicroscopy) can provide additional useful information.40 Biopsy forceps and cytology brushes are essential, and either balloon or Savary bougie dilators (see Chapter 17) should be available if dilation is necessary to facilitate endoscope passage and completion of the procedure.
Anatomy
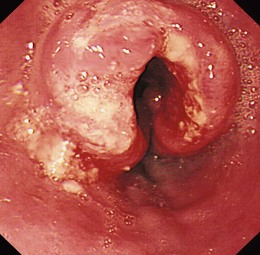
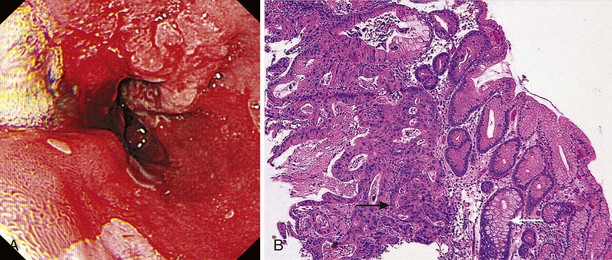
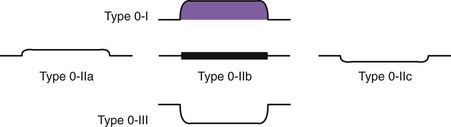
Early lesions may appear as minor irregularities of the mucosa; areas of erythema; or depressed, raised, or ulcerated areas (Figs. 25.5 and 25.6; see also Figs. 25.1 and 25.4). A high index of suspicion is required, and biopsy specimens should be obtained of any tissue with these abnormalities. A more recent study has suggested that characterization of early lesions in Barrett’s segments according to the Paris classification may offer useful information to aid decisions regarding the likelihood of submucosal invasion and the suitability for endoscopic mucosal resection (EMR) (see Figs. 25.4 and 25.6C). Elevated lesions (type 0-I) are associated with a higher prevalence of submucosal involvement, and ulcerated or depressed lesions (type 0-III) are technically unsuitable for EMR.41 This study also showed that most early cancers in Barrett’s esophagus were flat lesions that were difficult to visualize. Dye staining of the mucosa improves the detection rate and is discussed subsequently. More advanced lesions are usually obvious polypoid or ulcerating masses. Stenosis of the esophageal lumen may be present because of tumor bulk or involvement of the muscular layers. A tightly closed distal esophageal sphincter with normal mucosal appearance and resistance to the passage of the endoscope suggests pseudoachalasia.
Procedure
Chromoendoscopy
Lugol’s iodine reacts with glycogen in squamous epithelial cells to produce a uniform dark brown coloration. Inflamed, dysplastic, and malignant cells are glycogen-depleted and consequently appear minimally stained or unstained.42,43 Using a standard washing catheter inserted through the biopsy channel of the endoscope, 1% to 3% Lugol’s iodine is sprayed onto the mucosa. Most highly dysplastic or malignant lesions remain unstained (see Fig. 25.6), and clinical trials have shown that biopsy of these areas enhances detection of high-grade dysplasia and early carcinoma.44,45 The technique is simple and does not require specialized equipment or additional staff; it is described in more detail in Chapter 30.
Methylene blue is a vital stain taken up by the cytoplasm of absorptive cells. These include goblet cells present in Barrett’s epithelium, in addition to normal cells of the colon and small intestine.46 Methylene blue staining was first shown to stain selectively areas of specialized intestinal metaplasia in Barrett’s esophagus in 1996.47 Neoplastic change is associated with a reduction in goblet cell numbers, an increasing nuclear-to-cytoplasm ratio, and reduced absorption of methylene blue. Increasing grades of dysplasia may appear as heterogeneous or unstained areas, allowing targeted rather than random biopsy specimens to be taken.48,49 However, the emergence of electronic chromoendoscopy in the setting of Barrett’s esophagus has eclipsed methylene blue dye staining.
Biopsy and Cytology
Biopsy samples are obtained using forceps inserted via the biopsy channel of the endoscope. The cup volume of standard forceps is 12.4 mm3. The cup volume of the larger jumbo forceps is 30.4 mm3 allowing larger biopsy samples to be taken, but an endoscope with a 3.7-mm channel is required. A nonendoscopic balloon device for obtaining esophageal cytology has been developed in China mainly for use in screening high-risk populations.50
Jumbo forceps may provide better tissue samples for detection of high-grade dysplasia or early carcinoma in Barrett’s esophagus.51 Biopsy specimens are taken from the edge of ulcerated lesions to avoid necrotic tissue, and at least six samples are required to confirm the diagnosis in 100% of patients.52 Larger biopsy specimens can also be obtained by a “turn and suction” technique, in which the forceps are inserted, opened, and withdrawn until flush with the endoscope tip. The tip is turned onto the esophageal wall or lesion while suctioning air from the lumen; this draws tissue into the forceps, which are closed and withdrawn in the usual way.53
Brush cytology alone may detect malignancy and in some studies has been shown to improve the diagnostic yield when combined with standard biopsy.38,54 For best results, brushing should be performed before biopsy to minimize contamination with blood. Samples are obtained by passing the brush catheter into the lumen and drawing the brush across the lesion several times until minor mucosal bleeding is noted. Smears are made on slides and fixed in alcohol before staining using Papanicolaou’s technique.
Use of Esophageal Dilation
In 20% to 40% of cases, a malignant stricture prevents the passage of a standard adult endoscope.55 This situation may prevent both a complete examination and the performance of an adequate biopsy from within the main body of the tumor, but a cytology brush passed into the stricture may be a useful adjunct to obtain the biopsy sample from the proximal end of the lesion.56 Dilation facilitates biopsy, relieves symptoms, and enables subsequent staging with EUS. Biopsy immediately after dilation is safe57; for dilation of malignant strictures, the complications of hemorrhage and perforation occur in 2.5% to 10% of patients.58,59 Dilation provides only short-term palliation of malignant dysphagia, however, with the effects lasting days or at the most a couple of weeks. The issue of dilation before EUS staging is discussed later (see also Chapter 17).
Staging of Esophageal Carcinoma
Prognosis depends on tumor stage at diagnosis. Patients with early disease (T1N0M0; stage I) have 5-year survival rates greater than 90% with surgery alone, but prognosis worsens with advancing stage at diagnosis; patients with stage IV disease have 5-year survival rates of less than 5%. In patients without nodal involvement at surgery (N0), 5-year survival is approximately 40% to 60%, but 5-year survival is only 5% to 17% in patients with nodal involvement (N1). Patients undergoing surgery alone for T3N1 disease have 5-year survival rates of 8% to 10%,60,61 underlining the reality that although these tumors may be technically resectable, they are rarely curable. Accurate staging is essential in determining prognosis and in identifying patients for whom surgery alone is likely to be curative and patients with advanced disease for whom surgery has little to offer and for whom medical therapy or palliation should be the goal of therapy.
The benefits of neoadjuvant therapy have not yet been conclusively shown.62–64 A full discussion of the relative benefits of neoadjuvant therapy is beyond the scope of this chapter; however, given its increasing use and the considerable morbidity associated with it, it is imperative that patients undergo accurate staging to guide stage-specific therapy with the hope of improving survival. These strategies vary according to local practice and must be understood by clinicians staging such patients. In addition, to obtain clinically useful and important information from future trials of neoadjuvant therapy, it is essential that study groups are well matched, and highly accurate staging is crucial in this regard.