Chapter 57 Cystic neoplasms of the pancreas
Epidemiology, clinical features, assessment, and management
Overview
Cystic neoplasms of the pancreas have become a well-defined radiographic entity over the last decade. With increasing use of cross-sectional imaging, nearly 2.6 cystic lesions per 100 individuals are encountered per year (Brugge et al, 2004a; Gorin & Sackier, 1997; Kimura et al, 1995). These lesions have a broad histologic differential, which has been well characterized by Klöppel and Kosmahl (2001) and others (Table 57.1; Kosmahl et al, 2004). This differential includes benign nonneoplastic pancreatic pseudocysts and also cystic neoplasms, which comprise benign entities such as serous cystadenoma, premalignant cysts such as intraductal papillary mucinous neoplasms (IPMNs), and cystic lesions with invasive carcinoma. The ability to differentiate among neoplastic pancreatic cystic neoplasms without the need for resection continues to evolve. Serous cysts can often be differentiated from mucinous cysts by cross-sectional imaging and endoscopic ultrasound (EUS) with cyst fluid analysis (Allen et al, 2003, 2006; Allen & Brennan, 2007; Brugge et al, 2004b; Carpizo et al, 2008; Ferrone et al, 2009). However, diagnostic dilemmas continue, and the ability to predict future progression to malignancy within the premalignant group is currently limited (Lee et al, 2008).
Table 57.1 Classification of Cystic Neoplasms of the Pancreas
| Neoplastic | Nonneoplastic |
|---|---|
| Epithelial | Epithelial |
| Benign | |
| Nonepithelial | |
| Borderline | |
| Malignant | |
| Nonepithelial | |
| Benign neoplasm (i.e., lymphangioma) Malignant neoplasm (i.e., sarcoma) |
IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm
Modified from Kosmahl M, et al, 2004: Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 445(2):168-178.
As shown in Table 57.1, these lesions remain a diagnostic challenge. As their incidence increases, so does the differential diagnosis. With an increasing knowledge of the clinicopathologic variables predictive of malignancy and an improved understanding of the natural history of many of the common cystic lesions, management has changed to one of selective resection. Despite the improvement in diagnostic capability, there still remain a significant number of lesions that are indeterminate; in this setting the clinician must balance the risk of malignancy with the morbidity and mortality associated with pancreatic resection.
Clinicopathologic Variables
The majority of patients with pancreatic cysts will have nonneoplastic inflammatory pseudocysts that develop as a complication of acute pancreatitis (see Chapter 54; Brugge et al, 2004a; Cannon et al, 2009). A pseudocyst is a fluid collection that is devoid of an epithelial lining. Pseudocysts have been reported to develop in up to 50% of patients who have acute pancreatitis (Cannon et al, 2009; Grace & Williamson, 1993; O’Malley et al, 1985). Because of the frequency of pancreatitis, some studies have reported that pseudocysts comprise up to 85% of all cystic pancreatic lesions. Pseudocysts can be managed with observation, endoscopic drainage, or operative drainage (Cannon et al, 2009). The particulars of the management of patients with pancreatic pseudocysts are beyond the scope of this chapter.
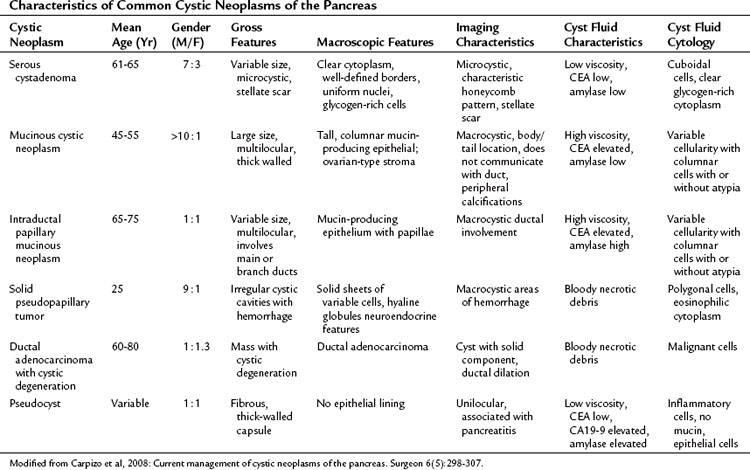
Cystic neoplasms are a heterogeneous group of lesions carefully described by Klöppel and Kosmahl (see Table 57.1; Klöppel & Kosmahl, 2001; Kosmahl et al, 2004). They comprise approximately 10% to 15% of cystic lesions of the pancreas and are a heterogeneous group of lesions that range from benign to malignant. Despite the fact that numerous neoplasms can present as a cystic lesion, the most common cystic neoplasms encountered by surgeons include serous cystadenoma (SCA), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN). In a study by the French Surgical Association of 372 resected cystic neoplasms from 73 institutions, these three lesions comprised 87% of all lesions (Le Borgne et al, 1999). More recent series of pancreatic cystic neoplasms are outlined in Table 57.2. The clinicopathologic factors associated with the more common cystic neoplasms are shown in Table 57.3.
Serous Cystadenomas
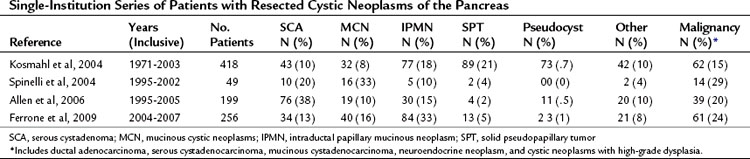
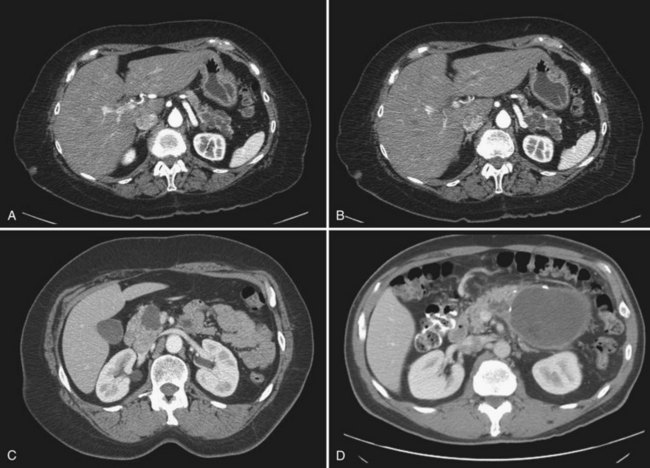
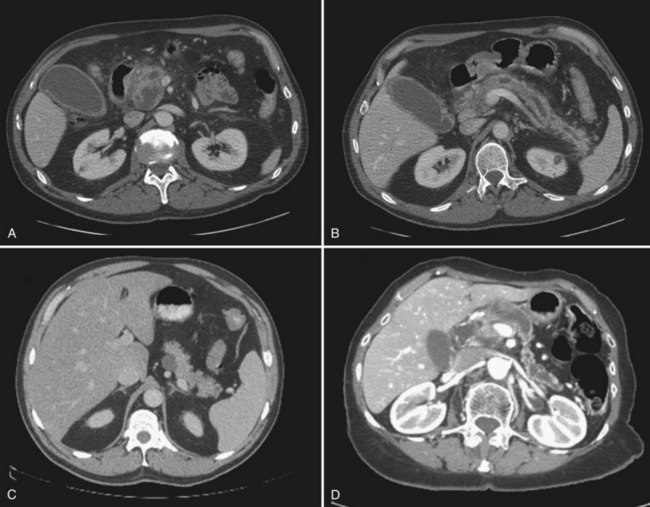
Serous cystadenomas (SCA) were first characterized by Compagno and Oertel in 1978 as microcystic and glycogen rich, and they distinguished these lesions from mucinous cysts (MCNs and IPMNs) (Compagno & Oertel, 1978a). SCAs occur with variable size, and when large (>10 cm) may cause symptoms from local compression. Grossly, these cysts are characterized by septations and thick fibrous walls and have what appear to be innumerable small cysts containing clear, thin fluid. A classic honeycomb appearance is often present, as is a calcified central scar with or without hemorrhage (Fig. 57.1A and B). On imaging, these lesions may appear as a single, large cyst (oligocystic) (Fig. 57.1C and D); if so, they are very difficult to distinguish from mucinous lesions. Because of the fibrous nature of this lesion, a solid component is often visualized; if noted in the presence of the other characteristic findings of SCA, this should not be concerning for malignancy. Histologically these lesions are characterized by a bland, cuboidal epithelial lining without nuclear polymorphism or mitoses. Typically, no mucin is present in the epithelial lining, but glycogen may be detected in the cytoplasm or in the amorphous cellular material.
SCAs are generally considered benign lesions, and only 25 cases of serous “cystadenocarcinoma” have been reported in the world literature as of 2010 (King et al, 2009; Matsumoto et al, 2005). Many of these case reports describe the “malignant” serous cystadenoma as locally invasive, and only 9 (36%) of 25 cases had evidence of metastasis. Despite the presence of metastasis, long-term prognosis remains excellent in these patients; it appears that the true incidence of malignancy within SCA is less than 1%. The institutional database at Memorial Sloan-Kettering Cancer Center (MSKCC) includes approximately 200 patients who have had resection for SCA, and none has developed or presented with metastatic disease. The more common problem caused by these lesions is local invasion that results in symptoms such as early satiety, obstructive jaundice, and pain. It stands to reason that large lesions are more likely to produce symptoms; however, many patients with lesions greater than 10 cm in diameter will have their lesion identified incidentally; for example, with a mean diameter of 11 cm, 71% of patients had symptoms compared with a mean diameter of 5 cm, with which 53% had symptoms (Compagno & Ortel, 1978a; Tseng et al, 2005).
Mucinous Cystic Neoplasms
Mucinous cystic neoplasms (MCNs) are mucin-producing cystic tumors that lack communication with the pancreatic duct and contain mucin-producing columnar epithelium. Ovarian-like stroma surrounding the columnar epithelium is considered a pathognomonic finding and is the presumed reason that MCNs are almost exclusively found in females. The stroma is a characteristic that pancreatic MCNs share with some mucinous cysts of the ovary and the liver. Pancreatic MCNs are most commonly found in the body and tail and can range in size from small (2 cm) to large (25 cm). Despite their distal location, patients are often symptomatic (76% in a review of multiple series) at the time of diagnosis (Goh et al, 2006).
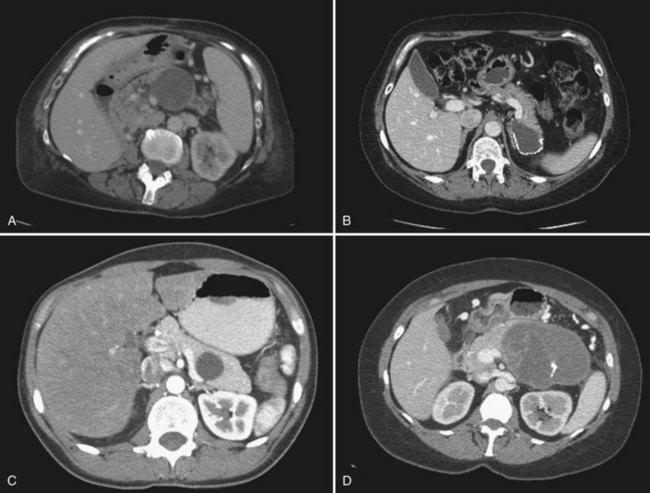
Any macrocystic lesion in the distal pancreas of a female patient should be highly suspicious for MCN. These lesions are often unilocular but may contain septa within the primary lesion (Fig. 57.2A to C). When these lesions are multilocular, they are typically macrocystic, in contrast to serous cystadenomas. These lesions may also have peripheral “eggshell” calcifications. Any evidence of mural nodularity in these lesions should be concerning for invasion (Fig. 57.2D). Upon gross inspection, these tumors are round, with a smooth surface and fibrous pseudocapsule.
Unlike serous cystadenomas, MCNs have a risk for harboring malignancy. Rates of invasive disease range from 10% to 50%, which may be an underestimate because of the fact that both benign and malignant epithelium may coexist within the same cyst. Only an extensive pathologic evaluation will detect both entities, which is critical for accurate diagnosis. Clinical factors that increase the risk for malignancy in MCNs are not well characterized, but one study found that older age was more often associated with cancer (Crippa et al, 2008). Factors on imaging associated with malignancy within MCNs include the presence of septations, larger size, and mural nodularity (Allen et al, 2006; Zamboni et al, 1999). Because these lesions are so rare, their natural history is not well defined, although the extent of invasion was demonstrated to be associated with recurrence and death from disease in one study (Allen et al, 2006; Zamboni et al, 1999). In one of the largest studies of MCNs to date, 5-year disease-specific survival for patients with malignancy was 57% (Goh et al, 2006).
Intraductal Papillary Mucinous Neoplasms
Before the first classification system published by the World Health Organization (WHO) (Klöppel et al, 1996), IPMN was described under a variety of names, including mucinous ductal ectasia, papillary carcinoma, and villous adenoma. In contrast to other mucinous and cystic tumors, IPMNs occur equally in men and women and are more often found in older individuals, with the peak incidence between 60 and 70 years of age (Brugge et al, 2004a). Among cystic neoplasms diagnosed in large series, IPMNs represent approximately 15% to 30% of all lesions (see Table 57.2; Allen et al, 2006; Ferrone et al, 2009; Kosmahl et al, 2004; Spinelli et al, 2004). Because of the recent evolution in the understanding of IPMNs, many early reports may include a mixture of patients with IPMNs and MCNs and should be interpreted with caution.
Current nomenclature as defined by the WHO divides IPMN into the categories of adenoma (low-grade dysplasia), borderline (moderate dysplasia), carcinoma in situ (high-grade dysplasia), and carcinoma (Furukawa et al, 2005). As clinical experience with IPMN increases, it is becoming more evident that the underlying process presents as a spectrum of neoplasia with significant variation (Schnelldorfer et al, 2008). The clinical and radiologic presentation, malignant potential, and disease-specific outcomes are highly variable. There also appears to be a progression from adenoma to intraductal carcinoma to invasive carcinoma that can be predicted based on molecular studies.
IPMN is indeed a process that involves the entire pancreas; however, radiographically detectable disease may involve the main duct alone, branch ducts alone, or both (mixed variant). The main duct variant of IPMN is typically seen with diffuse ductal dilation (Fig. 57.3). The presence of a discrete mass or solid component should be concerning for malignancy. Branch duct IPMNs, particularly when smaller than 3 cm, do not have any defining features. They may be unilocular, multilocular, and may be present without dilation of the main pancreatic duct. In the absence of septations, mural nodules, or a solid component, branch duct IPMNs may be indistinguishable from pancreatic retention cysts, MCNs, small cystic endocrine tumors, or even pancreatic pseudocysts.
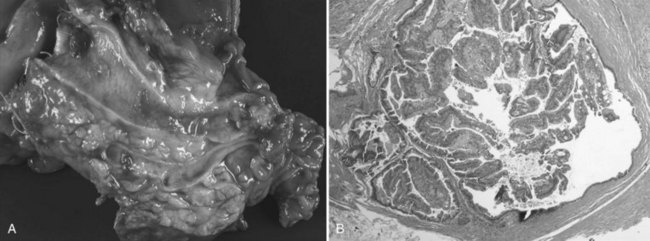
IPMNs are now a well-characterized mucinous cystic tumor of the pancreas evidenced by ductal involvement and mucin production. Recently, a histopathologic consensus conference defined IPMN as a grossly visible, noninvasive, mucin-producing neoplasm arising from either the main or side branches of the pancreatic duct with variable degrees of ductal dilation (Fig. 57.4A; Furukawa et al, 2005). On histologic analysis, IMPN lesions are characterized by papillary projections of columnar lined epithelium with varying degrees of dysplasia. Mucin is typically abundant both within the cytoplasm of the lining epithelial cells as well as within the acellular fluid matrix (Fig. 57.4B).