Fig. 13.1
Pharmacologic mechanism of calcineurin and mTOR inhibitors. Similar mechanisms of action group cyclosporine, tacrolimus, and sirolimus into dependent inhibitors (see Chap. 14 for further information). Each is capable of negating the action of a transcription factor/activator by cooperative binding with either cyclophilin or FK506. Importantly, these attenuating mechanisms reside within the immunologic regulation of T cells
Rescue Therapy
Immunomodulation of the inflammatory microenvironment with calcineurin inhibitors has been used as salvage therapy for steroid-refractory UC patients. It is thought that “refractoriness” derives from an overwhelming proinflammatory milieu that reduces the anti-inflammatory affinity of steroids to its receptor and attenuates its effects. A patient is defined as refractory to steroids when their use has not decreased UC symptoms (increased stool urgency, frequency, hematochezia, colonic dilation, tenesmus, fever) within 3–5 days. It is important for the clinician to exclude the presence of pseudo-refractory states that are not appropriately managed with the use of corticosteroids prior to initiating rescue therapies. These would include symptoms related to the presence of adhesions, fibrotic intestinal strictures, abscesses, small intestinal bacterial overgrowth, Clostridium difficile-related colitis, opportunistic viral infections (cytomegalovirus), or lactose intolerance. These are clinical scenarios that simulate the presence of UC-like symptoms often indistinguishable from those in patients with actual, active ulcerative colitis.
After a patient is deemed truly refractory and documented to have active UC, options to further treatment include immunomodulators such as azathioprine (AZA), 6-mercaptopurine (6-MP), and mycophenolate; the calcineurin/mTOR inhibitors tacrolimus and cyclosporine (CSA); and biologic agents like infliximab, adalimumab, and, more recently, golimumab [20].
The use of azathioprine (AZA) or 6-mercaptopurine (6-MP) is not typically used acutely and is not particularly helpful for patients with active, refractory UC given the long duration of time that is required for the onset of action. Two commonly prescribed calcineurin inhibitors include tacrolimus and cyclosporine, both of which downregulate T cell activation and chemokine production without significant myelosuppression (Fig. 13.1). Specifically, the calcineurin inhibitors bind to immunophilins and inhibit the calcineurin-dependent dephosphorylation and activation of nuclear factor of activated T cells (NFAT) [21]. Whereas tacrolimus attaches to the FK506 binding protein, cyclosporine binds to cyclophilin, and both ultimately inhibit NFAT transcription of differentiation, growth, and chemokine genes. Cyclosporine harbors a long history of clinical use as an antirejection drug in solid-organ transplantation as well as in rheumatoid arthritis [22]. It has a quick onset of action that results in severe UC improvement within 1–2 weeks at a dose between 2 and 4 mg/kg/day via continuous intravenous (IV) infusion [23]. Recent evidence suggests oral formulations may be as effective as IV administration and is described below.
Two other medications, sirolimus and mycophenolate mofetil, target immune cell proliferation but are not currently recommended as a standard of therapy for patients with UC pending clinical trials. Sirolimus also binds to the immunophilin FK506 binding protein but instead downregulates mammalian target of rapamycin (mTOR) attenuating T cell proliferation [21]. Mycophenolate mofetil acts as a prodrug that is hydrolyzed to mycophenolic acid, inhibiting the inosine monophosphate dehydrogenase enzyme. As such, guanine nucleotide synthesis and proliferation are downregulated specifically in B and T cells as they are incapable of rescue purine synthesis. Of note, only tacrolimus and cyclosporine harbor enough clinical data to warrant their recommended use in severe, steroid-refractory UC.
Cyclosporine Pharmacology
A fungal metabolite, cyclosporine is a lipophilic, cyclic peptide that is poorly soluble in water and must be either in an emulsion or a suspension prior to oral or IV use [24]. As such, there is a very narrow therapeutic window where levels below specific blood concentrations do not assist in attenuating the immunologic response, while levels above advance adverse effects. Additionally, the IV pharmacokinetic profile is variable and highly dependent on the patient’s cytochrome P450 profile within the liver, gut, and kidney as well as bile excretion dynamics. Importantly, high bile excretion (after a high-fat meal) will aid in the bioavailable absorption of cyclosporine but also in its excretion [25, 26].
As the cyclosporine microemulsion increases contact with the plasma, its pharmacokinetic profile is less variable and more regulated. This variation reaches peak plasma levels in approximately 2 h but has a highly variable half-life ranging between 9 and 27 h [27]. Its hepatic cytochrome P450 metabolism is similar to IV cyclosporine and its excretion also occurs mainly via bile excretion. After oral ingestion of cyclosporine, plasma levels achieve a maximal level (Cmax) in an average of 4 h, while its plasma half-life can reach 19 h [25, 28].
Cyclosporine Clinical Trials
The first landmark randomized, double-blind, placebo-controlled prospective trial assigned 20 patients with severe UC not improving after at least 7 days of IV corticosteroids (equivalent to 300 of IV hydrocortisone or equivalent dose in other formulations) to receive either IV cyclosporine at 4 mg/kg/day (n = 11) or placebo (n = 9) for up to 14 days [29]. Response was defined as improvement on a numerical Lichtiger scale from 0 (no symptoms) to 21 (severe symptoms) with a score of less than 10 on 2 consecutive days [29]. The active treatment arm had 9/11 (82 %) of patients with a validated response within a mean of 7 days when compared to 0/9 (0 %) of patients in the placebo arm (p < 0.001) [29]. Nonresponders, two patients in cyclosporine arm and four patients in placebo arm, underwent colectomy, while five remaining nonresponders in the placebo arm crossed over to open-label treatment with IV cyclosporine [29]. In all five placebo patients that crossed over to IV cyclosporine, a clinical response was observed within a mean of 7 days with a decrease in their mean Lichtiger score from 11 to 7 [29]. Importantly, the mean disease activity index within the treatment group was decreased by more than 50 %, permitting all cyclosporine-treated patients to have successful hospital discharges [29]. The small number of enrolled patients in this study was in part due to the hospital’s safety committee stopping the trial early due to the observation of statistically significant responses in the active treatment group [29]. The initial trial was planned with the intent to enroll 42 total patients.
A subsequent randomized, double-blind controlled trial published in 2001 observed that IV cyclosporine had comparable efficacy to IV methylprednisolone alone in severe UC flares [20]. There were 29 patients who were randomly assigned to an 8-day course of either IV cyclosporine (4 mg/kg/day) or IV methylprednisolone (40 mg/day) [20]. Patients who demonstrated responses at day 8 (defined as a Lichtiger score of less than 10 on days 7 and 8 with a decrease in the Lichtiger score from day 1 to day 8 of at least three points and the possibility to discharge the patient) received the same medication orally in an open-label fashion (cyclosporine 8 mg/kg or methylprednisolone 32 mg/day) combined with oral azathioprine 2–2.5 mg/kg/day [20, 29]. Oral methylprednisolone was given at a dose of 32 mg/day for the first 3 weeks with a subsequent taper by 4 mg/week until discontinuation, whereas oral cyclosporine was continued for 3 months and then discontinued [20]. Oral azathioprine was continued for up to 12 months [20].
After the initial 8 days of IV therapy, 8/15 (53 %) of patients on methylprednisolone and 9/14 (64 %) of patients on cyclosporine had a response (p = 0.4) to therapy without severe, drug-related toxicity observed, suggesting similar efficacy of cyclosporine and glucocorticosteroids in severe attacks of UC [20].
Further, 7/9 (78 %) of patients with a cyclosporine-induced response maintained UC remission at 12 months on oral azathioprine when compared to 3/8 (37 %) of patients with a methylprednisolone-induced response [20]. Overall, 1-year colectomy rates were 36 % (5/14 patients) in the cyclosporine group and 40 % (6/15 patients) in the methylprednisolone group [20]. Cyclosporine was shown to be an efficacious alternative to glucocorticosteroids in inducing a response in patients with severe UC and also as a bridging agent to oral azathioprine after achievement of a clinical response [20].
Of further clinical importance, applying cyclosporine to UC patients while attenuating steroid exposure can benefit patients who are sensitive to avascular necrosis, osteoporosis, or immune deficiency.
A single-center, randomized double-blind controlled trial compared the efficacy and safety of an 8-day treatment with IV cyclosporine 4 mg/kg versus IV cyclosporine 2 mg/kg in patients with an acute, severe UC flare [30]. Following the Lichtiger clinical activity index as described above [29], 73 patients with a severe UC flare were enrolled and followed for 8 days on either the 4 mg/kg or 2 mg/kg IV cyclosporine dosage [30]. The following concomitant medications were allowed: (1) IV corticosteroids (stable dose for at least 5 days without clinical response prior to enrollment and during the 8 days of the trial), (2) oral corticosteroids (initiated at least 14 days from inclusion without clinical benefit) which were switched to IV corticosteroids on day 1 of the trial with subsequent transition to oral corticosteroids on day 8 with a taper by 5 mg of prednisone per week, (3) AZA/6-MP (started at least 3 months prior to inclusion with a stable dose 4 weeks prior to admission), (4) both oral and rectal aminosalicylates (continued at stable doses for the first 8 days), and (5) antibiotics (continued at inclusion if clinically necessary and during the trial for infections) [30]. Of note, patients who were not on azathioprine at the time of trial onset started receiving azathioprine 2–2.5 mg/kg orally on day 8 [30].
Clinical response rates (defined as a Lichtiger clinical activity index (CAI) score less than 10 at day 8 with a drop of at least three from baseline) were 84.2 % for the 4 mg/kg arm and 85.7 % for the 2 mg/kg arm (p > 0.05) with a median time to clinical response of 4 days in both arms, signifying that the lower dose was as efficacious as the higher [30]. Coordinately, the blood levels of cyclosporine correlated with their dosing amounts such that 2 mg/kg had a blood level of 237 ± 33, while 332 ± 43 ng/mL was observed in the 4 mg/kg group (p < 0.0001) [30]. Short-term colectomy rates were not statistically significantly different between the 4 mg/kg group and the 2 mg/kg group (13.1 % vs. 8.6 %, p > 0.05) [30]. The multivariate logistic regression analysis determined that, from several variables such as active smoking, mean cyclosporine dose, patient’s age, location of UC (left-sided vs. pancolitis), and concomitant corticosteroids and azathioprine use, only active smoking was inversely associated with clinical response (OR 0.06, 95 % CI 0.008–0.407) [30].
There were no statistically significant differences between the treatment arms (4 mg/kg vs. 2 mg/kg) in the proportion of patients experiencing adverse events such as tremor or paresthesia (7.9 % vs. 5.7 %, p-value not reported), increase of serum creatinine by at least 10 % (18.4 % vs. 17.1 %, p-value not reported), fever (7.9 % vs. 2.9 %, p-value not reported), or diabetes mellitus (2.6 % vs. 0 %, p-value not reported) [30]. However, a trend toward a greater proportion of novel hypertension in the higher cyclosporine arm was observed (23.7 % vs. 8.6 %, p < 0.08) [30]. It was suggested that lower doses of cyclosporine should be used in patients with acute, severe UC given the comparable efficacy to higher doses and better safety profile [30]. There was a suggestion that active smokers with severe UC may become refractory to all medical treatment, but the small number of smokers in this study precludes the definitive interpretation of this finding [30].
A retrospective cohort analysis examined 142 patients admitted to a tertiary medical center with an acute, severe UC flare. These patients were stratified to either treated with IV cyclosporine (2–4 mg/kg/day) in conjunction with IV glucocorticosteroids for 7 days after they deteriorated or not responding to 5–7 days of prior treatment with IV glucocorticosteroids (methylprednisolone 40 mg/day) [31]. Patients whose condition worsened or did not improve while on IV cyclosporine for 7–10 days underwent immediate colectomy [31]. Those patients who responded to IV cyclosporine (83 %, 118/142 patients) were then switched to a tapering dose of oral glucocorticosteroids and 3 months of oral cyclosporine emulsions (Neoral, Novartis) at an initial dose of 8 mg/kg/day that was adjusted to blood cyclosporine levels ranging between 150 and 250 ng/mL, with addition of azathioprine (2.5 mg/kg/day) or 6-mercaptopurine (1.5 mg/kg/day) [31]. Of the 142 patients, 44 were already on azathioprine at the time of their severe flare, 74 were started on azathioprine de novo, and 24 patients did not receive azathioprine/6-mercaptopurine [31]. However, it is unclear when azathioprine de novo was initiated as the authors initially stated it occurred after achieving responses to IV cyclosporine with subsequent statements noting that azathioprine was initiated at the time of onset of IV cyclosporine therapy [31].
Among 118 patients who avoided initial colectomy, 41 (35 %) underwent a future colectomy within a mean of 542 days [31]. According to the life table analysis, overall 1-year and 7-year colectomy rates were 33 % and 88 %, respectively [31]. Subgroup analysis showed that the proportion of patients who underwent colectomy was statistically and significantly (p < 0.05) greater among those patients who were already on azathioprine (59 %, 26/44 patients) at the time of the severe flare when compared to those who were started de novo on azathioprine at the time of treatment with IV cyclosporine (32 %, 24/74 patients). This observation suggests that prior failure of azathioprine to maintain a state of remission predicted poor treatment success with cyclosporine for severe UC flares [31]. In other words, patients who failed prior therapy with azathioprine and who presented with severe activity mandating the use of cyclosporine had poorer outcomes than those individuals who were azathioprine naïve at the time they received the cyclosporine for refractory disease (Fig. 13.2). Furthermore, of the 26 patients who were already on azathioprine and required colectomy, 23 (88 %) underwent colectomy within 1 year after an initially successful treatment with cyclosporine. This can be compared to a 50 % 1-year colectomy rate (12/24 patients) in the subgroup of patients who were started on azathioprine de novo and required colectomy [31].
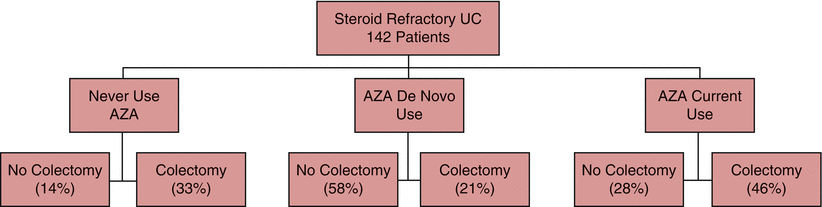

Fig. 13.2
Cyclosporine-responsive patients without prior thiopurine usage have the highest success of avoiding colectomy. Stratifying by chronology of azathioprine dosing (never with cyclosporine (de novo) or before cyclosporine (current)). Patients naïve to AZA and co-dosed with cyclosporine at the same time avoid colectomy at the highest rate (58 %) compared to those on cyclosporine who do not receive AZA (14 %) or those who had prior AZA therapy prior to cyclosporine treatment (28 %) [31]
The authors concluded that cyclosporine is indeed effective in the short term and that AZA-naïve patients show better outcome prior to beginning cyclosporine therapy [31]. It was suggested that IV cyclosporine should be used as a bridge to long-term treatment with immune modulators or colectomy.
Further, a prior retrospective study including 42 patients with severe UC treated with IV cyclosporine 4 mg/kg/day for a mean of 10 days and 31 patients continuing oral cyclosporine at 8 mg/kg/day for a mean of 20 weeks showed that the combination of cyclosporine and azathioprine/6-mercaptopurine was associated with a significantly higher probability of avoiding colectomy at 5.5 years than cyclosporine monotherapy (66 % vs. 40 %, p = 0.04) [32]. Colectomy-free rates were 62 % for all patients, 72 % for responders to cyclosporine, and 80 % for responders to cyclosporine on concomitant azathioprine/6-mercaptopurine. Further, life table analysis demonstrated colectomy-free rates at 5.5 years of 58 %, 70 %, and 71 %, respectively [32]. The results of both studies should be interpreted with caution due to their retrospective design and low number of patients enrolled [31, 32].
Recent meta-analysis of six retrospective cohort studies comparing treatment with infliximab versus cyclosporine in 321 patients with acute, severe corticosteroid-refractory UC demonstrated comparable therapeutic profiles of both agents in terms of rescue therapy [33]. No statistically significant difference was observed in the 3-month (OR = 0.86, 95 % CI 0.31–2.41, p = 0.775) and 12-month colectomy rates (OR = 0.60, 95 % CI = 0.19–1.89, p = 0.381), in adverse drug reactions (OR = 0.76, 95 % CI = 0.34–1.70, p = 0.508), or in postoperative complications (OR = 1.66, 95 % CI = 0.26–10.50, p = 0.591) between infliximab and cyclosporine [33]. These data were further supported by a recent multicenter, parallel, open-label randomized controlled trial designed by GETAID in which 115 cyclosporine and infliximab naïve patients from 27 medical centers in Europe presenting with severe UC refractory to IV corticosteroids were randomly assigned to receive either IV cyclosporine at the dose of 2 mg/kg/day for 1 week followed by oral cyclosporine at the daily dose of 4 mg/kg for 91 days (goal trough 150–250 ng/mL, n = 58) or infliximab infusions at the dose of 5 mg/kg on days 0, 14, and 42 (n = 57) [34]. All patients were maintained on stable doses of IV corticosteroids for 7 days and then switched in responders to oral methylprednisolone (30 mg/day) with subsequent taper [34]. In addition, those with a clinical response at day 7 were started on azathioprine 2–2.5 mg/kg/day or were continued on azathioprine if it was initiated within 4 weeks before trial onset [34]. Primary efficacy end points included treatment failure defined as presence of any of six predefined criteria: (1) no clinical response within the first 7 days, (2) clinical relapse (increase in Lichtiger score by at least three points sustained for 3 consecutive days) between days 7 and 98, (3) absence of corticosteroid-free remission on day 98 (Mayo disease activity index of less than 2 and an endoscopically defined subscore of less than 1), (4) interruption of treatment secondary to severe adverse events, (5) need for colectomy, or (6) patient’s death [34]. The proportion of patients who experienced treatment failure was similar between the two treatment arms (60 % in cyclosporine arm vs. 54 % in infliximab arm, p = 0.52) [34]. Multivariate analysis adjusted for independent predictors of treatment failure (age greater than 40 years and hemoglobin concentration 95–125 g/L) showed a nonsignificant increased odds ratio for treatment failure with cyclosporine versus infliximab at 1.4 (95 % CI 0.6–3.2, p = 0.36) [34]. The authors suggested that given comparable efficacy, treatment choice with either cyclosporine or infliximab should be based on the physician’s or medical center’s experience [34]. Furthermore, data from a small retrospective study of 19 patients with severe corticosteroid-refractory UC who were treated with IV cyclosporine after failing to respond clinically to infliximab or with infliximab after failing to respond clinically to IV cyclosporine suggested that cyclosporine and infliximab might be efficacious salvage agents for each other in this patient population [35]. In that study, remission was achieved by 40 % of patients receiving infliximab salvage therapy with mean duration of 10.4 months and 33 % of patients receiving cyclosporine salvage therapy with mean duration of 28.5 months [35]. Caution should be exercised when implementing this strategy immediately after failure of one agent and reserved after a “resting period” to avoid infectious complications resulting from massive immunosuppression [19].
A prospective study of 83 consecutive patients presenting with corticosteroid-refractory severe UC who received salvage therapy with either IV cyclosporine (n = 45) or infliximab (n = 38) showed that 84 % of patients who received a single dose of infliximab (5 mg/kg) versus 56 % of patients who received at least 72 h of IV cyclosporine (2–4 mg/kg/day) avoided colectomy (p = 0.006) [36]. Similarly, the proportions of patients who avoided short-term and medium-term colectomy were significantly greater in those treated with infliximab when compared to oral cyclosporine at 3 months (76 % vs. 53 %, p = 0.04) and 12 months (65 % vs. 42 %, p = 0.04), respectively [36]. In addition, the only two adverse events occurred in patients receiving cyclosporine [36]. However, serious adverse event rates of 16 % (3/19 patients) indicated that the risk of using this agent as salvage therapy may outweigh the benefits [35].
The most recently published retrospective cohort study of 78 patients with severe corticosteroid-refractory UC who underwent colectomy following treatment with IV corticosteroids alone or combined with either IV cyclosporine or infliximab at a tertiary university center suggested that neither cyclosporine nor infliximab was associated with an increased risk of postoperative complications [37]. No difference in total postoperative complications was observed between patients who received cyclosporine (RR = 0.63, 95 % CI 0.33–1.23) or infliximab (RR = 0.65, 95 % CI, 0.36–1.17) in conjunction with IV corticosteroids and those receiving IV corticosteroid monotherapy [37]. Furthermore, no significantly increased risk of infectious (cyclosporine with IV corticosteroids, RR = 0.54, 95 % CI, 0.17–1.76; infliximab with IV corticosteroids, RR = 0.86, 95 % CI, 0.36–2.09) or noninfectious (cyclosporine with IV corticosteroids, RR = 0.88, 95 % CI, 0.43–1.80; infliximab with IV corticosteroids, RR = 0.40, 95 % CI, 0.15–1.07) postoperative complications was observed in patients treated with cyclosporine or infliximab combined with IV corticosteroids when compared with IV corticosteroids alone [37].
According to a recent systematic review of the literature, remission rates achieved with IV cyclosporine were 91.4 % in four controlled trials and 71.4 % in 18 uncontrolled trials with the lower 2 mg/kg/day dose being safer and as efficacious as the higher, standard 4 mg/kg/day dose [38]. The Cochrane meta-analysis on the efficacy of cyclosporine in severe UC published in 2005 [23] was not able to provide pooled data due to significant differences in design and patient populations in the two randomized controlled trials [20, 29] that were included in the final analysis. Further, they suggested that there is limited evidence supporting superiority of efficacy with short-term treatment with cyclosporine than standard treatment alone for severe UC [23]. It was also suggested that long-term treatment with cyclosporine has unclear benefits due to the risk of adverse events, in particular nephrotoxicity [23]. Recent data from studies comparing cyclosporine and infliximab in severe UC suggest that there is not enough strong evidence to prefer one agent over the other and that results from ongoing randomized controlled trials will likely help to determine the best agent for medical salvage therapy [39]. In the end, early discussions of benefits of surgery with your patient are recommended due to the fact that a delay in offering surgery to a patient may increase the risk of complications, and this population is recognized to carry a 2.8 % mortality given the fact that they have already failed first-line treatment options [39].
There is consensus that cyclosporine therapy should be initiated by experienced physicians who are faced with patients unresponsive to corticosteroids, within the initial week of the flare, and tailored to symptomatology and blood tests.
Analysis of Long-Term Cyclosporine Therapy
Small prospective and larger retrospective analyses have analyzed the long-term side effects of cyclosporine therapy prior to subsequent relapse or colectomy [40, 41]. Campbell et al. constructed the largest retrospective database of 76 patients with acute corticosteroid-refractory UC who required treatment with either IV (4 mg/kg/day) or oral (5 mg/kg/day) cyclosporine for a median of 4 days or 4 weeks [40]. Patients who responded (<3 bowel movements/day and C-reactive protein <45 mg/L) to IV or oral cyclosporine rescue therapy underwent long-term treatment with oral cyclosporine for a median of 6 weeks with an initial daily dose of 5 mg/kg that was later titrated to a trough blood level of 150–300 ng/L and were followed for a median of 2.9 years [40]. As soon as patients’ corticosteroids were discontinued, treatment with oral azathioprine was initiated concomitantly with oral cyclosporine [40]. Although, overall, an initial remission was achieved by 74 % (56/76) of patients, only 35 % of initial responders maintained their remission by 12 months and 10 % by 36 months, and only 42 % of initial responders were colectomy-free after 84 months of follow-up [40]. Neither duration of treatment with IV hydrocortisone prior to treatment with cyclosporine nor addition of azathioprine to cyclosporine improved time to first relapse or time to surgery [40]. On the other hand, time to the first relapse (p < 0.01) and time to the colectomy (p < 0.05) were significantly greater in patients treated with oral cyclosporine when compared to IV [40].
These data were further supported by a recent retrospective analysis of the records of 36 patients (38 episodes of cyclosporine use) presenting with acute corticosteroid-refractory UC who, within a median of 8 days after hospitalization, were primarily started on oral cyclosporine (32/38 episodes) at the initial dose of 4.5–8.3 mg/kg/day (six patients were started on IV cyclosporine 2–5 mg/kg/day and switched to oral formulation after 2–8 days). These data showed that 30.5 % (11/36) of the patients immediately failed to respond to cyclosporine within a mean of 6.1 days and underwent colectomy [41]. Among 25 patients who initially responded to cyclosporine and were subsequently discharged on oral cyclosporine (85 % were started on azathioprine within a median of 5 weeks after discharge), 84 % were colectomy-free after a median follow-up of 3.8 years [41].
Another small cohort study evaluated 23 patients treated with oral microemulsion cyclosporine (5 mg/kg/day titrated to blood trough concentration 200 ng/mL) for 3 months due to corticosteroid-refractory or corticosteroid-dependent UC and demonstrated a 70 % clinical response (at least 50 % reduction in clinical activity) rate (16/23 patients) at 3 months [42]. Those who responded to oral cyclosporine were switched to oral azathioprine at the daily dose of 2 mg/kg for a median of 24 months, while nonresponders underwent colectomy [42]. After a median follow-up of 12 months among initial responders, 11 patients were colectomy-free, while 5 underwent colectomy with a chronic response rate of 47 % (11/23 patients) [42]. The study also compared their results with data from the five largest published studies. Their series evaluated 210 patients with ulcerative colitis treated with IV cyclosporine followed by oral formulation with an acute response of 68 % and a chronic response of 42 % [32, 42–46]. It was therefore proposed that the oral microemulsion formulation of cyclosporine may replace IV cyclosporine in treating patients with acute, severe corticosteroid-refractory or corticosteroid-dependent UC [42]. These observations suggest that cyclosporine therapy may have long-term successful outcomes in avoiding colectomy when doses and blood levels are monitored and adverse effects are dealt with on a patient-by-patient basis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






