Variables
Overall
n = 293
WL < 5 %
n = 205
WL = 5–6.99 %
n = 34
WL = 7–9.99 %
n = 25
WL ≥ 10 %
n = 29
P value**
Weight loss (%)
3.8 ± 2.7
1.78 ± 0.16
5.86 ± 0.09
8.16 ± 0.22
13.04 ± 6.6
–
Resolution of steatohepatitisa
72 (25)
21 (10)
9 (26)
16 (64)
26 (90)
<0.01
NAS improvementb
138 (47)
66 (32)
21 (62)
22 (88)
29 (100)
<0.001
– Change in NAS from baseline
−1.58 ± 0.27
−0.89 ± 0.13
−1.94 ± 0.36
−3.84 ± 0.29
−4.10 ± 0.23
<0.001
Steatosis improvement
142 (48)
72 (35)
22 (65)
19 (76)
29 (100)
<0.001
– Change from baseline
−0.63 ±0.10
−0.36 ± 0.07
−1 ± 0.13
−1.40 ± 0.19
−1.69 ± 0.12
<0.001
Lob. Inflammation improvement
147 (50)
72 (35)
24 (71)
22 (88)
29 (100)
<0.001
– Change from baseline
−0.49 ± 0.15
−0.29 ± 0.05
−0.53 ± 0.22
−1.32 ± 0.09
−1.21 ± 0.11
<0.001
Ballooning improvement
115 (39)
54 (26)
14 (41)
21 (84)
26 (90)
<0.001
– Change from baseline
−0.45 ± 0.17
−0.24 ± 0.04
−0.41 ± 0.13
−1.12 ± 0.13
−1.34 ± 0.08
<0.001
Fibrosis status c
<0.01
– Regression
56 (19)
33 (16)
6 (18)
4 (16)
13 (45)
– Stabilized
191 (65)
129 (63)
25 (74)
21 (84)
16 (55)
– Worsened
46 (16)
43 (21)
3 (8)
0 (0)
0 (0)
– Change from baseline
−0.01 ± 0.02
0.09 ± 0.07
−0.02 ± 0.03
−0.17 ± 0.12
−0.86 ± 0.20
<0.001**
Portal inflammation improvement
44 (15)
27 (13)
3 (9)
5 (20)
9 (31)
0.049
– Change from baseline
0.02 ± 0.02
0.06 ± 0.01
0.09 ± 0.03
−0.07 ± 0.01
−0.31 ± 0.08
<0.01**
NAS
<0.001
NAS ≤ 2
119 (41)
48 (23)
20 (59)
22 (88)
29 (100)
NAS 3–4
79 (27)
74 (36)
2 (6)
3 (12)
0 (0)
NAS ≥ 5
95 (32)
83 (41)
12 (35)
0 (0)
0 (0)
Adequately powered studies addressing the role of macronutrient composition in managing NAFLD are sparse [19, 24]. A recent report suggested a greater reduction in hepatic triglycerides as measured by magnetic resonance spectroscopy (−55 ± 14 % versus −28 ± 23 %) following 2 weeks of carbohydrate-restricted diet as compared to 2 weeks for calorie-restricted diet despite a similar degree of weight loss with both diets (−4.6 ± 1.5 kg versus −4.0 ± 1.5 kg) [10]. Whether these changes in hepatic fat content are sustainable over time with these different diets is currently unknown. There are however data on the long-term sustainability of weight loss with different diets. A large randomized trial using four different hypocaloric diets with emphasis on different macronutrients showed no significant differences between diets including those with higher or lower carbohydrate portions after 2 years of dietary modification [25]. This supports the current multi-society practice guidelines recommendation of hypocaloric diet to achieve weight loss without specific emphasis on any macronutrient [22].
While increased dietary intake of fructose particularly in corn syrup has been suggested to contribute to the increased risk of NAFLD and even more severe NAFLD histology [26–28], more recent reports have challenged this proposition. Although increased consumption of carbohydrates including fructose correlated with obesity risk, fat and total energy intake had more influence on intrahepatic triglycerides content [29, 30].
The benefit of exercise as part of the recommended lifestyle modification for NAFLD patients has been demonstrated in several studies [5, 7, 11, 13, 14, 16, 31–33]. The degree of physical fitness correlates with the risk of NAFLD, with lower fitness strongly correlating with presence of NAFLD and increased fitness correlating with resolution of fatty liver by magnetic resonance spectroscopy [32]. Furthermore, aerobic and resistance exercise training may improve insulin sensitivity and hepatic steatosis independent of weight loss [11, 14, 31, 34].
The limited long-term durability of weight loss achieved through lifestyle modification represents a practical limitation to this approach as an effective strategy to manage NAFLD. Most of the weight loss is seen in the first 6 months of these interventions and may reach up to 10 % of initial body weight. One study randomized 811 subjects to 4 types of reduced caloric diets with variation in carbohydrate, protein, and fat content for 2 years [25]. At 6 months, the average weight loss was 6 kg, and many subjects began regaining weight after the first year. By 2 years, the average weight loss was 4 kg, and only 15 % of the subjects were able to lose more than 10 % of their baseline weight. Their macronutrient composition did not affect the degree of weight loss among the different study groups.
A systematic review evaluated 80 randomized trials of different weight loss modalities (total of 24,698 subjects, 68 % completed the planned studies) that had a minimum of 1 year follow-up [35]. Weight loss was achieved through diet or exercise alone, diet and exercise, meal replacements, very-low-energy diets, medications (orlistat or sibutramine), or advice alone. After an initial average weight loss of 5–8.5 kg, weight plateaued at 6 months. Only an average of 3–6 kg (3–6 %) of the weight loss was maintained at 4 years (Fig. 16.1). No significant weight loss was achieved in subjects who received advice or exercise alone.
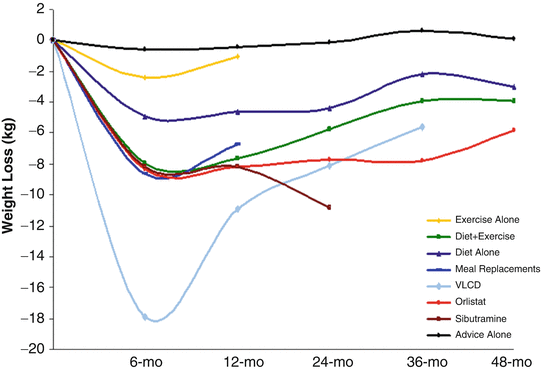

Fig. 16.1
Long-term weight loss with various types of nonsurgical methods. Courtesy Dr. Marion Franz. Reproduced Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67 [35], with permission of the American Dietetic Association
On the other hand, morbidly obese patients who have a high prevalence of NAFLD experience sustainable weight loss reaching up to 25 % of initial body weight at 5 years following bariatric surgery [36]. A recent systematic review of bariatric surgery studies with at least 2 years of follow-up showed that both gastric bypass and sleeve gastroplasty consistently resulted in a minimum of >50 % excess weight loss, an outcome observed only in 31 % of gastric banding studies [37]. All types of bariatric procedures resulted in improvement in associated comorbidities including type 2 diabetes, dyslipidemia, and hypertension [37]. The effects of bariatric surgery on NAFLD have been reported in numerous studies and summarized in recent meta-analysis and systematic review [36, 38–46]. The majority of patients with NAFLD experience improvement or resolution of steatosis and steatohepatitis (Fig. 16.2). Improvement in fibrosis including resolution of cirrhosis has been reported, but some studies reported an increase in mild fibrosis in 7–40 % of the patients [41, 44, 47–49].
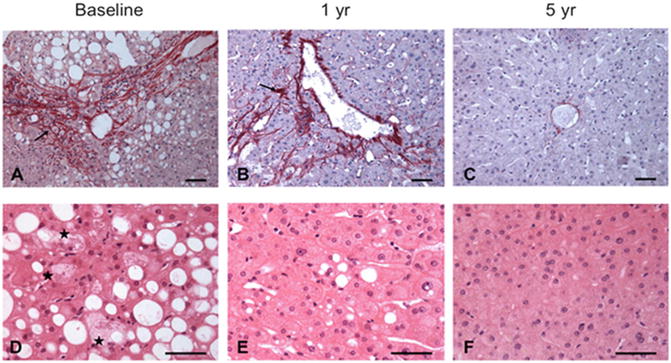

Fig. 16.2
Resolution of both steatohepatitis and significant fibrosis following Roux-en-Y gastric bypass surgery. Courtesy Dr. Francois Pattou. Reproduced from Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260(5):893–9 [36], with permission of Wolters Kluwer Health
A careful look at the five studies reporting worsening fibrosis (Table 16.2) reveals that three of these studies used wedge biopsies which tend to be associated with increase in peripheral fibrosis and frequently lead to overestimation of fibrosis [47–49]. The remaining two studies came from the same group and reported an increase in fibrosis at 1 and 5 years following bariatric surgery [41, 44]. Liver histology was evaluated with core liver biopsies, and a considerable proportion of patients (42 %) underwent biliointestinal bypass in the first study [41], a procedure known to be associated with increased risk for development of hepatic fibrosis [50]. In the second study from this group, biliointestinal bypass was performed in 23 % of the patients [44]. About 80 % of patients had fibrosis regression, whereas 20 % experienced fibrosis progression at 5 years following bariatric surgery. In this cohort, >90 % of patients with fibrosis progression went from stage 0 to 1; and in the total cohort with follow-up biopsies, 96 % had fibrosis stage ≤1, and 0.5 % had stage 3. These patients remained morbidly obese at 5 years following surgery (BMI 40.5 ± 8.3 kg). Only one patient who underwent biliointestinal bypass progressed to cirrhosis in the setting of alcohol abuse. On multivariate analysis, only underlying fibrosis but not BMI, steatosis, inflammation, or ballooning influenced the progression. In this study, the investigators reported no significant differences in fibrosis progression between the gastric band, biliointestinal, and gastric bypass groups between baseline and 5 years. However, the investigators just published another follow-up report detailing their 5-year follow-up of their bariatric cohort [36], in which they declared that their center abandoned biliointestinal bypass due to concerns about association with liver fibrosis and replaced it with adjustable gastric band, Roux-en-Y gastric bypass, or sleeve gastrectomy. In this updated report, 13 patients had bridging fibrosis at baseline, which regressed in six and disappeared in two patients. There was no report of worsening fibrosis in this cohort without biliointestinal bypass from the same center. Roux-en-Y gastric bypass resulted in more weight loss and improvement in NAFLD histology in this report although patients were not randomized and the type of surgery was chosen by patients. Patients who received the adjustable gastric band had lower BMI, HOMA, and NAS scores at baseline. Based on these data, there is no convincing evidence that weight loss induced by Roux-en-Y gastric bypass, gastric band, or sleeve gastrectomy is associated with progression of hepatic fibrosis.
Table 16.2
Summary of bariatric surgery studies reporting increase in hepatic fibrosis
Author | N | Baseline BMI (kg/m2) | Procedure | Baseline fibrosis | Number with repeat biopsy | Interval to repeat histology (months) | Method of liver biopsy | Fibrosis evolution | Comments |
|---|---|---|---|---|---|---|---|---|---|
Kral et al. [48] | 689 | 47 ± 9 | Biliopancreatic diversion | 14 (2 %) with baseline cirrhosis | 104 | 41 ± 25 | Wedge | Increased 40 %, decreased 27 % Stable 33 % | Severe fibrosis decreased in 28, whereas mild fibrosis appeared in 42 11 patients with cirrhosis had decreased fibrosis by 2 stages 7 patients had disappearance and 2 regression of nodules and fibrous bridging |
Stratopoulos et al. [49] | 51 | 52.8 ± 1 | Vertical band gastroplasty | F0 6 % F1 33 % F2 45 % F3 6 % F4 0 % | 51 | 18 ± 9 | Wedge | F0 14 % F1 49 % F2 27 % F3 10 % F4 0 % | 16 subjects had a third liver biopsy 17 ± 6 months after the second biopsy showing significant reduction fibrosis |
Csendes et al. [47] | 557 | 44.3 | Roux-en-Y gastric bypass | Only 1 with any fibrosis and had cirrhosis | 16 | 17 | Wedge | 1 with new stage 1 fibrosis Stable cirrhotic but with resolution of NASH | |
Mathurin et al. [41] | 171 | 49 ± 8 | Gastric band (58 %) Biliointestinal bypass (42 %) | F0 81 % F1 10 % F2 2 % F3 1 % F4 6 % | 121 | 12 | Core needle | Mean fibrosis score, increased from 0.14 ± 0.39 to 0.38 ± 0.64 % patients without significant fibrosis <2 was not significantly different between the preoperative and the postoperative period: (98.6 % vs. 95 %) | Increase in mean fibrosis score not clinically relevant Bariatric surgery not done in 14 patients with suspected cirrhosis |
Mathurin et al. [44] | 376 | 50 ± 8 | Gastric band (56 %) Biliointestinal bypass (23 %) Gastric bypass (21 %) | F0 77 % F1 18.5 % F2 4 % F3 0.5 % F4 0 % | 211 | 60 | Core needle | Fibrosis regression 80 % progression 20 % Fibrosis progression mainly from stage 0 to1 In the total cohort with follow-up biopsies, 96 % had fibrosis stage ≤1 and 0.5 % had stage 3 | Patients with fibrosis progression remained morbidly obese at 5 years following surgery (BMI 40.5 ± 8.3 kg) One patient who underwent biliointestinal bypass progressed to cirrhosis in the setting of alcohol abuse |
Bariatric surgery can therefore be an option to NAFLD patients with morbid obesity [22]. There is however paucity of data about the safety and optimal type of bariatric surgery for patients with NASH-related cirrhosis, with the few case series including patients with cirrhosis reporting improvement in NASH histology and regression of cirrhosis in many patients [47, 48].
Vitamin E
It has long been contended that oxidative stress contributes to the pathogenesis of NASH [51–56]. Based on its known function as an antioxidant [57], vitamin E has been used alone or with other compounds in multiple trials to treat NASH or NAFLD [1, 58–66]. Varying dosages ranging from 100 to 1200 IU/day have been used with reported beneficial effects on liver enzymes, steatosis, inflammation, ballooning, and hepatic fibrosis. The duration of vitamin E monotherapy in these trials ranged from 4 to 96 weeks [65, 67]. Vitamin E effects on ALT could be seen in as early as 4 weeks [65] and on histology in 6 months [60, 61]. In one of the largest randomized trials in NASH to date, the PIVENS [63], vitamin E was given at dose of 800 IU/day for 96 weeks and compared to pioglitazone or placebo. Vitamin E and pioglitazone improved ALT, steatosis, lobular inflammation, and ballooning and resulted in resolution of NASH in a significant number of patients. There was no improvement in fibrosis. Vitamin E, however, was not associated with weight gain as pioglitazone. This trial excluded patients with NASH if they had diabetes or cirrhosis. In the TONIC clinical trial [66], which compared the efficacy of vitamin E to that of metformin in children with biopsy-proven NAFLD, significant improvement in ballooning grade and NAFLD activity score was noted only with vitamin E. Other trials with vitamin E monotherapy were small, and either did not include diabetics or included a small subgroup of diabetics.
A meta-analysis by Miller et al. raised concerns about an increase in all-cause mortality with vitamin E use [68]. Although other meta-analyses confirmed this finding [69, 70], these results were contested by other analyses which did not show an association but raised the possibility of underlying patients’ condition as a possible cause of increased mortality associated with vitamin E intake [71, 72]. More recently, an increased rate of prostate cancer was reported in a trial with vitamin E administered to healthy men [73]. The multi-society practice guidelines recommend considering vitamin E at 800 IU daily as a first-line therapy for patients with histologically confirmed NASH without cirrhosis or type 2 diabetes [22].
Thiazolidinediones
The peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists , rosiglitazone and pioglitazone , improve insulin sensitivity by decreasing glucose production and increasing its utilization in the muscle, adipose, and liver tissue. Thiazolidinediones (TZDs) induce favorable effects on production of adiponectin, resistin, interleukin-6, and tumor necrosis-alpha and reduce circulating free fatty acids, thus modulating inflammation and atherosclerosis [74–77]. Pioglitazone also has a PPAR-α effect which mediate hepatic fatty acid oxidation and inflammation [78].
Several small studies evaluated the effects of rosiglitazone on NAFLD [79–84]. Initial reports suggested improvement in all NASH histological features. However, the FLIRT2 trial examined the long-term effects of extending the use of rosiglitazone for 2 additional years in subjects who already participated in an initial 1 year placebo-rosiglitazone trial [82]. The prolonged use of rosiglitazone in this trial was only associated with continued improvement in ALT, insulin sensitivity, and steatosis, but not hepatic necroinflammation or fibrosis.
The beneficial effects of pioglitazone on NAFLD have also been shown in several clinical trials [60, 63, 85–87]. In the PIVENS clinical trial, pioglitazone resulted in NASH resolution in 34 % of the subjects compared to 43 % of subjects on vitamin E and 19 % for the placebo group. Pioglitazone significantly improved steatosis and necroinflammation, but not fibrosis in nondiabetic, non-cirrhotic subjects with NASH in this trial [63]. A recent meta-analysis of data from this study in addition to two prior trials of pioglitazone in NASH [86, 87] confirmed the improvement in steatosis and necroinflammation but also showed improvement in fibrosis in the pooled data (Fig. 16.3) [88].
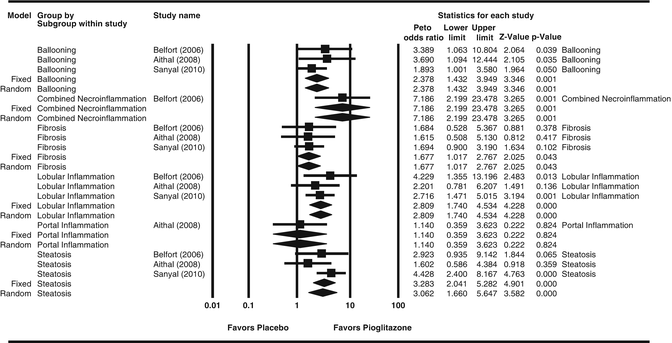

Fig. 16.3
Effects of pioglitazone on liver histological features in nonalcoholic steatohepatitis. Courtesy Dr Rohit Loomba. Reproduced from Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75 [88], with permission of John Wiley and Sons
Except for increased weight (4.7 kg over the study 96-week period), pioglitazone was well tolerated and did not have higher adverse events compared to placebo in this trial.
However, the metabolic and histological improvements seen with pioglitazone use reverse upon its discontinuation. In a follow-up study of 21 patients after 48 weeks of pioglitazone therapy, 13 subjects were followed for an additional 48 weeks, and 9 subjects had repeated liver biopsy [89]. Rebound increases in ALT and HOMA, decrease in adiponectin level, and worsening steatosis and inflammation were observed. This study not unexpectedly shows that long-term use of pioglitazone is necessary to maintain the associated metabolic and hepatic benefits.
While the majority of patients with NASH in the TZD trials were nondiabetics, there is continuing controversy about the TZDs long-term safety for treating type 2 diabetes. Reports of increased incidence of bone fractures, congestive heart failure, and bladder cancer have raised concern about TZDs safety [90, 91]. The cardiovascular profile for pioglitazone may be better than that of rosiglitazone as it was associated with lower risk for cardiovascular events, including congestive heart failure and myocardial infarction, in a recent meta-analysis [92]. These different cardiovascular effects were postulated to be related to the different effects pioglitazone exerts on metabolic genes in addition to observed improvements in triglycerides and high-density lipoprotein cholesterol levels compared to rosiglitazone.
The current multi-society practice guidelines recommend that pioglitazone may be used with caution in nondiabetic patients with biopsy-proven NASH [22].
Obeticholic Acid
Since the discovery of the bile acid nuclear receptor farnesoid X receptor (FXR) [93, 94], there has been considerable progress in the understanding of its biological functions. FXR plays an essential role in regulating bile acids synthesis and transport, lipid and glucose homeostasis, and hepatic inflammation [95, 96]. Obeticholic acid (OCA) is a semisynthetic derivative of the primary human bile acid chenodeoxycholic acid, the natural agonist of the FXR. Based on its potent and selective FXR agonist effects, OCA has been tested as a potential therapeutic agent for NASH in two clinical studies. The initial study was a pilot human trial of short duration consisting of 64 patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus [97]. Patients were randomized to receive placebo, 25 mg OCA, or 50 mg OCA once daily for 6 weeks. The primary end point of the study was improvement in insulin sensitivity as measured by the hyperinsulinemic-euglycemic clamp technique. Changes in liver enzyme levels were among several measured secondary end points. Compared to placebo, OCA improved insulin sensitivity, ALT, and Enhanced Liver Fibrosis test , a validated serum marker of fibrosis. Importantly, treatment was associated with weight loss. Despite remaining within the normal range, there was a mild increase in alkaline phosphatase levels in subjects receiving OCA compared to placebo.
This study was followed by a large multicenter randomized clinical trial (FLINT) of oral OCA in patients with NASH without cirrhosis [98]. In total, 283 patients with biopsy-proven NASH and NAFLD activity score (NAS) of ≥4 were randomized to receive OCA 25 mg orally daily or matching placebo for 72 weeks. Nearly 50 % of the subjects had type 2 diabetes. The primary outcome of this study was improvement in liver histology defined as a decrease in NAS by at least 2 points without worsening of fibrosis from baseline to the end of treatment. Due to its vanguard study design, after a planned interim analysis showed significant beneficial effects of OCA on histology, treatment was ended early in the last 64 subjects and a follow-up liver biopsy was not obtained for them. A greater number of participants receiving OCA met the primary study outcome on OCA as compared to placebo (45 % versus 21 %, relative risk 1.9, 95 % CI 1.3–2.8; p = 0.0002). There was significant improvement noted in steatosis, lobular inflammation, ballooning, and fibrosis with OCA (Table 16.3). Although resolution of definite NASH was observed more frequently with OCA than placebo (22 versus 13 %), this did not reach statistical significance (p = 0.08).
Table 16.3
Effects of 72 weeks of obeticholic acid therapy on liver histology in patients with NASH
Obeticholic acid | Placebo | Relative risk or change Obeticholic acid vs. placebo | ||
|---|---|---|---|---|
(95 % CI) | P-value | |||
Primary outcome | ||||
No. of patients at riska | 110 | 109 | ||
Patients with improvement—n (%) | 50 (45.5) | 23 (21.1) | 1.9 (1.3, 2.8) | 0.0002 |
Changes from baseline in histologic features | ||||
No. of patients with biopsy specimens at baseline and 72 weeks | 102 | 98 | ||
Resolution of definite nonalcoholic steatohepatitis—n (%) | 22 (21.6) | 13 (13.3) | 1.5 (0.9, 2.6) | 0.08 |
Fibrosis b | ||||
Patients with improvement—n (%) | 36 (35.3) | 19 (19.4) | 1.8 (1.1, 2.7) | 0.004 |
Change in score—mean ± SD | −0.2 ± 1.0 | 0.1 ± 0.9 | −0.3 (−0.6 to −0.1) | 0.01 |
Total NAFLD activity score | ||||
Change in score—mean ± SD | −1.7 ± 1.8 | −0.7 ± 1.8 | −0.9 (−1.3 to −0.5) | <0.0001 |
Hepatocellular ballooning | ||||
Patients with improvement—n (%) | 47 (46.1) | 30 (30.6) | 1.5 (1.0, 2.1) | 0.03 |
Change in score—mean ± SD | −0.5 ± 0.9 | −0.2 ± 0.9 | −0.2 (−0.5 to 0.0) | 0.03 |
Steatosis | ||||
Patients with improvement—n (%) | 62 (60.8) | 37 (37.8) | 1.7 (1.2, 2.3) | 0.001 |
Change in score—mean ± SD | −0.8 ± 1.0 | −0.4 ± 0.8 | −0.4 (−0.6 to −0.2) | 0.00041 |
Lobular inflammation | ||||
Patients with improvement—n (%) | 54 (52.9) | 34 (34.7) | 1.6 (1.1, 2.2) | 0.006 |
Change in score—mean ± SD | −0.5 ± 0.8 | −0.2 ± 0.9 | −0.3 (−0.5 to −0.1) | 0.0006 |
Portal inflammation | ||||
Patients with improvement—n (%) | 12 (11.8) | 13 (13.3) | 1.0 (0.6, 1.7) | 0.90 |
Change in score—mean ± SD | 0.2 ± 0.7 | 0.2 ± 0.7 | 0.0 (−0.1 to 0.2) | 0.59 |
Pruritus occurred more commonly with OCA (23 % versus 6 %). OCA therapy was associated with a decrease HDL and increase in total and LDL cholesterol at 12 weeks of therapy. These changes attenuated with therapy and resolved after discontinuation of OCA. Similar to the earlier OCA study, there was mild increase in alkaline phosphatase in the OCA group. There was an average weight loss of 2.3 kg with OCA compared to no weight loss in the placebo group. Five severe or life-threatening events that were deemed related to OCA including three events of severe pruritus, one of hyperglycemia, and one of possible cerebral ischemia. There were two deaths in subjects receiving OCA and were deemed not related to the study drugs (one from myocardial ischemia and another from sepsis and heart failure).
The favorable effects of OCA on liver histology are encouraging. Additional studies to validate the findings and provide longer-term follow-up will be needed to confirm efficacy and define the safety profile of this agent.
Liver Transplantation
Liver transplantation is a therapeutic option for patients with NASH cirrhosis who develop liver failure or hepatocellular carcinoma. Indeed, there has been a considerable increase in liver transplantation for NASH over the past decades. NASH is currently the second most common indication for liver transplantation in the USA and is projected to become the leading indication in the next one to two decades [99–102]. Patients with NASH cirrhosis who are listed for liver transplantation are usually older and have higher BMI and lower incidence of HCC than those listed for other indications [99, 103]. Despite a more complex transplantation course marked by increased intraoperative blood loss, longer operative times, and posttransplant length of stay [101], the 1- and 3-year posttransplant survival for patients transplanted for NASH cirrhosis are excellent (average 85 % and 78 %, respectively); they are at least comparable to that autoimmune disease and better than hepatocellular carcinoma, hepatitis C virus, alcoholic liver disease, acute hepatic necrosis, and hemochromatosis [99–101, 104]. Although NAFLD and NASH recur following transplantation in up to 40 % of the patients, graft and patient survival are not affected at least in the short to midterm [101, 105–107]. Meticulous selection of candidates with NASH cirrhosis for liver transplantation is necessary for optimal outcomes posttransplantation. Given the high prevalence of cardiovascular disease in patients with NAFLD, potential candidates should undergo a thorough pre-transplant cardiac evaluation to diagnose and treat underlying cardiac disease [108]. Optimization of the metabolic syndrome and obesity management is also an important aspect of the care in the pre- and posttransplantation setting [109].
Other Agents
Metformin
Metformin is a commonly used hypoglycemic agent. It inhibits hepatic gluconeogenesis, enhances peripheral tissue utilization of glucose, reduces circulating free fatty acids, and decreases food intake and body weight, changes that are collectively associated with improved insulin sensitivity [110–112].
Metformin has been tested as a treatment for NAFLD or NASH in several trials. Initial small studies reported improvement in ALT, steatosis by imaging, and liver histology with metformin [113–119]. In the largest clinical trial in adults [113], 110 nondiabetic Italian subjects with NAFLD (mean age 43 years, BMI 28.8 kg/m2) were randomized to receive metformin (2 g/day; n = 55) versus vitamin E (800 IU/day; n = 28) versus dietary intervention to reduce weight (n = 27) for 12 months. ALT improved in all cases. In the 17 subjects who received metformin and agreed to repeat liver biopsy, significant improvement in hepatic steatosis, necroinflammation, and fibrosis was observed. However, metformin effects on liver histology could not be reproduced in several subsequent studies [66, 120–122]. In the largest randomized trial testing the effects of metformin to date, the TONIC clinical trial [66], 173 children (aged 8–17 years) with biopsy-confirmed NAFLD were randomized to receive vitamin E (800 IU/day, n = 58), metformin (1000 mg/day, n = 57), or placebo (n = 58) for 96 weeks. Neither metformin nor vitamin E showed significant difference compared to placebo in achieving sustainable decrease in ALT. Reduction in ballooning grade and NAFLD activity score, and increase in proportion of patients with NASH resolution with both metformin and vitamin E, reached statistical significance only with vitamin E, but not metformin therapy. There was no significant improvement in the other NAFLD histological features with either therapy compared to placebo. A meta-analysis that pooled the results from four studies with metformin found no effect for metformin on liver histology in patients with NAFLD [123]. Based on this data, the multi-society practice guidelines do not recommend metformin for the treatment of liver disease in patients with NASH [22].
Ursodeoxycholic Acid
Ursodeoxycholic acid (UDCA) has many attractive putative mechanisms of actions that prompted testing it as a potential therapy for NAFLD. In addition to altering the bile acid pool, UDCA has choleretic, anti-inflammatory and anti-apoptotic effects and may modulate immune response and mitochondrial integrity [124].
Earlier studies reported improvement of ALT and steatosis in patients with NAFLD with UDCA at a daily dose of 12–15 mg/kg alone or when combined with vitamin E [62, 64, 125]. Another study using high-dose UDCA (28–35 mg/kg/day for 12 months) in 126 subjects with biopsy-proven NASH showed reduction in ALT together with improvement in serum markers of insulin resistance and hepatic fibrosis [126]. However, two large randomized studies in patients with biopsy-confirmed NASH at baseline and with histological end points failed to show significant effects for UDCA on NASH histology with low-dose (13–15 mg/kg/day for 2 years, n = 166) and high-dose (23–28 mg/kg/day for 1.5 years, n = 185) UDCA [127, 128]. This data does not support the use of UDCA for treatment of NAFLD or NASH.
Statins
The hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase) inhibitors, also known as statins, are widely used for the treatment of dyslipidemia and primary and secondary prevention of cardiovascular disease [129, 130]. Several small reports suggest that these agents are safe when used in patients with NAFLD and may result in decreasing ALT levels and hepatic steatosis on imaging [131–135]. Two small studies suggested an improvement in histological features other than fibrosis, but no histological changes were noted in a third study [136–138]. Based on this inconclusive data and lack of adequately designed randomized trials, statins cannot be recommended as treatment for NASH.
However, in patients with NAFLD or NASH, statins can be safely used to treat dyslipidemia as demonstrated by the above studies and data from larger studies specifically looking at their safety in the setting of NAFLD and other liver diseases [139–141]. In a large study that evaluated the safety of statins in dyslipidemic patients with underlying liver disease, there was no significant difference in the incidence of elevated liver tests of varying severity after 6 months of use between subjects with and without elevated baseline liver enzymes [140]. In a randomized controlled trial of high-dose pravastatin in patients with compensated chronic liver disease and dyslipidemia [141], the incidence of ALT elevation to more than twice the upper limit of normal at the end of 36-week trial was lower in subjects who received pravastatin compared to placebo (7.5 % vs. 12.5 %), although this did not reach statistical significance (p = 0.13). Another large study (the GREACE study) showed that in patients with underlying coronary artery disease and elevated transaminases presumably due to NAFLD, those who received statin (88 % received atorvastatin) experienced an improvement in their liver tests compared to those who did not [139]. More importantly, they suffered lower number of cardiovascular events compared to those with elevated liver tests who did not receive a statin (10 % vs. 30 %, p < 0.0001). Less than 1 % of study subjects discontinued statin due to elevation in transaminase to more than three-times the upper limit of normal per study protocol. All together, these data suggest that there is no evidence for increased severe hepatotoxicity in patients with NAFLD who receive statins.
Fibrates
Fibrates are commonly used to treat hypertriglyceridemia [77]. By activating PPAR-α, fibrates increase hepatic fatty acid oxidation and reduce hepatic triglyceride synthesis and VLDL production and export [142]. In patients with the metabolic syndrome, fibrates reduce plasma triglyceride, C-reactive protein, and interleukin-6 without affecting hepatic or peripheral insulin sensitivity or circulating free fatty acids [143, 144].
There are a few small studies exploring the effects of fibrates on NAFLD with conflicting fibrates effects on ALT [125, 145, 146]. One study showed no effect of 8 weeks therapy with fenofibrate on hepatic fat content despite decreasing plasma triglycerides and VLDL [147]. Two studies reported fibrates effect on liver histology in NAFLD with one showing improvement only in ballooning, while the other reported no improvement in histology [125, 146]. Based on current available data, fibrates cannot be recommended to treat NASH.
Long-Chain Polyunsaturated Fatty Acids
Long-chain polyunsaturated fatty acids (LC-PUFA) in the n-3 (omega-3: ω-3) series including docosahexaenoic acid (DHA; C22:6n-3) and eicosapentaenoic acid (EPA; C20:5n-3) are abundant in fish and fish oil supplements and exert several beneficial biological effects. In addition to lowering triglycerides and increasing HDL, LC-PUFA increase circulating adiponectin, improve insulin sensitivity, and reduce body weight, adipose tissue inflammation, endothelial dysfunction, and coronary artery disease risk [148–150]. These effects are highly desirable in patients with NAFLD in whom cardiovascular disease is the leading cause of death. Based on analysis of the National Health and Nutrition Examination Survey (NHANES) 2003–2008 data, a majority of US adults do not consume the recommended daily amount of LC-PUFA [151]. There is also data to suggest the LC-PUFA desaturation is altered in NASH with imbalance between the pro-inflammatory (ω-6 pathway) and the anti-inflammatory (ω-3) pathways [152].
LC-PUFA have been tested as treatment of NAFLD in several studies with reports of improved ALT and hepatic steatosis by imaging [153–158]. These studies were limited either by small size, non-biopsy diagnosis of NAFLD, or design issues. A meta-analysis of data pooled from these studies showed significant heterogeneity among studies but yielded consistent effect for LC-PUFA on improving hepatic steatosis [159]. The pooled data could not confirm improvement in transaminases with this therapy. A recent study randomized 37 diabetic patients with NASH to receive a combination of EPA+DHA (4 g/day) or placebo containing corn oil for 48 weeks [160]. There was no change in liver enzymes or histology with LC-PUFA in this study. In a randomized placebo-controlled study of 103 subjects with NAFLD (the WELCOME study), 15–18 months treatment with DHA+EPA (4 g/day) did not result in significant decrease in hepatic fat content or serum markers of fibrosis [161]. The lack of effect was attributed to compliance in the treatment arm and contamination with DHA/EPA in the placebo group. There was a significant correlation noted between the increase in erythrocytes enrichment with DHA and decreasing hepatic fat content. In a large multicenter trial of EPA, 243 subjects with biopsy-proven NASH were randomized to receive placebo, EPA 1800 mg/day, or EPA 2700 mg/day for 12 months. EPA therapy had no effects on ALT, liver histology, or serum levels of keratin-18, hyaluronic acid, C-reactive protein, or insulin resistance [162]. Finally, in a recent study in 41 patients with NASH but without cirrhosis, subjects were randomized to receive 3 g of n-3 fish oil or placebo for 12 months [163]. Treatment with fish oil had no significant effects on NASH histological lesions compared to placebo. Subgroup analysis of subjects who maintained or increased their weight during the study showed a nonsignificant trend for reduction in liver fat by morphometric and MRI quantifications in the treatment arm but not by the standard semiquantitative scoring of steatosis on liver biopsy. To date, no study has yet explored the efficacy of purified DHA alone as a potential therapy for NASH. This approach is attractive because experimental data suggest DHA may be more potent than EPA in suppressing hepatic lipogenesis, inflammation, oxidative stress, and fibrosis [164, 165].
Angiotensin Antagonists
There is evidence for a role of the renin-angiotensin system in regulating hepatic stellate cells and fibrogenesis. Activation of the angiotensin II receptor 1 results in stellate cells activation and proliferation, whereas blocking this receptor or angiotensin II results in stellate cell apoptosis and diminished hepatic fibrosis [166–168]. In a cross-sectional retrospective study, the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) in patients with NAFLD and hypertension was associated with milder degree of fibrosis and ballooning [169]. One study randomized 150 patients with NAFLD to receive either losartan or amlodipine for 6 months followed by simvastatin [170]. Losartan resulted in reduction in hepatic steatosis, visceral adipose tissue, and insulin resistance compared to amlodipine, and these effects were further enhanced by adding simvastatin. In another study of 54 patients with NASH and hypertension, patients were randomized to receive one of two ARBs, valsartan or telmisartan for 20 months [171]. ALT, HOMA, and liver histology improved in both groups. Both drugs resulted in significant reduction in steatosis, but only telmisartan significantly improved lobular inflammation, ballooning, fibrosis, and the NAFLD activity score.
Pentoxifylline
Pentoxifylline is a nonselective phosphodiesterase inhibitor that has many putative functions including suppression of tumor necrosis factor-α, increasing hepatic glutathione, and reducing hepatic inflammation and oxidation of free fatty acids [172–174].
It has been tested as a treatment for NASH in a few small studies. Earlier reports suggested reduction of ALT [175, 176]. One small open-label study reported improvement in NAFLD activity score with pentoxifylline [177]. Similar results were shown in a randomized controlled trial of 55 patients with NASH, with improvement in steatosis, lobular inflammation, and fibrosis, but not ballooning after 1 year of pentoxifylline therapy [178]. However, another randomized study of 30 patients with NASH did not show significant improvement in NASH histology compared to placebo [179]. Additional large clinical trials are needed to test pentoxifylline’s effects of NASH histology.
Emerging Therapies
Simtuzumab
Lysyl oxidases (LOX) are a family of extracellular matrix cross-linking enzymes involved in cross-linking collagen and elastin. Simtuzumab is a humanized monoclonal antibody to LOX like (LOXL) 2 [180]. Two clinical trials in patients with NASH and bridging fibrosis (NCT01672866) or cirrhosis (NCT01672879) are currently evaluating its safety and effects on hepatic venous pressure gradient, hepatic fibrosis, and overall and hepatic event-free survival.
GR-MD-02
Galectin 3 protein is important in hepatic fibrogenesis. GR-MD-02 is a complex carbohydrate galectin 3 inhibitor that improved fibrosis and portal hypertension in toxin-induced cirrhosis and resulted in regression of fibrosis in a murine model of NASH with fibrosis [181, 182]. The results of an early phase 1 trial have recently been presented [183] and demonstrated safety of this compound in patients with NASH and bridging fibrosis. This compound reduced serum markers of fibrosis (FibroTest® and Keratin-18) and inflammatory markers (tumor necrosis factor-alpha and interleukin-6 and 8) in studied subjects. A multicenter, phase 2 study of GR-MD-02 in patients with NASH cirrhosis is underway in the USA.
Exenatide
Exenatide is a synthetic glucagon-like peptide-1 (GLP-1) agonist. It exerts strong regulatory effect on postprandial insulin secretion and glucose metabolism [184].
There is early data from animal and human studies showing that exenatide reduces free fatty acid-induced endoplasmic reticulum stress and apoptosis [185] and results in improvement in hepatic steatosis in patients with type 2 diabetes by improving sensitivity to fibroblast growth factor 21 [186–188]. In a small open-label study, eight patients with diabetes and biopsy-proven NASH received exenatide for 28 week [189]. There was improvement in NASH histological lesions which resulted in reduction in the NAFLD activity score in five subjects. Fibrosis improved by one stage in three subjects and by two stages in one and worsened by one stage in one subject, while it remained stable in the other three subjects. Large randomized controlled trials are needed to adequately assess exenatide effects on NASH histology.
Fibroblast Growth Factor 21
Fibroblast growth factor 21 (FGF21) is a member of the hormone-like FGF subfamily and is a potent regulator of metabolism and energy homeostasis [190, 191]. In murine models of NAFLD and NASH, FGF21 administration has been shown to improve hepatic steatosis, inflammation, and fibrosis [192–194]. In Ossabaw miniature swine with diet-induced NASH, FGF21 administration resulted in improvements in hepatic necroinflammation and fibrosis, insulin sensitivity, and postprandial lipidemia [195]. No clinical trials in humans have yet been undertaken to evaluate the effects of FGF21 on NASH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






