No trials of adequate power have compared the efficacy of the newer 5-ASA drugs and sulfasalazine. However, several studies have compared 5-ASA to glucocorticoid therapy for induction of remission (see Table 11.1). Schölmerich randomized 62 patients to receive either 5-ASA at a dose of 2g/day or a standard tapering regimen of methyl prednisolone [9]. In this 24-week trial 73% of the 5-ASA-treated patients failed therapy compared with 34% of those who received methyl prednisolone (ARR 39%, NNT = 3; p = 0.0019). Alc The authors concluded that treatment with 5-ASA, although well tolerated, was inferior to steroid therapy. Martin et al. compared a 3g/day dose of Salofalk to a standard oral prednisone regimen [10]. Although a similar proportion of individuals in the two treatment groups entered remission (47% 5-ASA vs 46% prednisone; p = 0.59), an analysis of the change in mean CDAI and quality of life scores demonstrated a more rapid improvement in patients treated with prednisone. A study by Thomsen and colleagues provides important information on the relative efficacy of glucocorticoids and 5-ASA [12]. In this methodologically rigorous trial 182 patients with active disease were assigned to receive either 9mg/ day of a controlled ileal release preparation of budesonide (a locally active steroid) or 4g/day of Pentasa. Following 16 weeks of treatment, 62% of budesonide treated patients were in remission compared with only 36% of the patients who received 5-ASA (ARR 26%, NNT = 4, p < 0.01). Ald
What conclusions can be drawn from these trials? The existing data show that the newer 5-ASA compounds are not more effective than sulfasalazine and are, at best, only marginally superior to a placebo for the induction of remission. A single clinical trial has demonstrated the superiority of budesonide over high dose 5-ASA, with no increased frequency of adverse events. Although many clinicians prescribe 5-ASA compounds as first-line therapy for mild disease activity and treat those patients who fail to achieve a remission with glucocorticoids, the wisdom of this approach is questionable. Although the reluctance of physicians to expose individuals to glucocorticoid therapy is understandable, the likelihood of a response to the newer 5-ASA formulations is so low that the strategy is inefficient. Most patients will ultimately require the more potent agents discussed below to induce remission. In any event, if a 5-ASA drug is used for induction of remission in patients with mild disease the best evidence supports the use of sulfasalazine. Alc
Table 11.1 Response rates of remission in studies comparing 5-ASA to placebo or glucocorticoid therapy.
Source: Feagan B. EurJ Surg 1998; 164: 903–909.
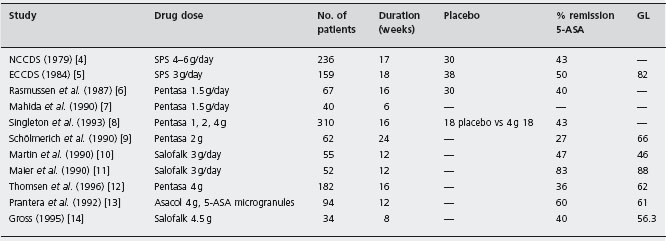
SFS: sulfasalazine; GL: glucocorticoids; 5-ASA: 5-aminosalicylate; NCCDS: National Cooperative Crohn’s Disease Study; ECCDS: European Cooperative Crohn’s Disease Study.
Glucocorticoids
Conventional steroids
The conventional glucocorticoid compounds, prednisone and 6-methyl prednisolone, are highly effective drugs for the treatment of active CD. The National Cooperative Crohn’s Disease Study (NCCDS) and the European Cooperative Crohn’s Disease Study (ECCDS) both showed that approximately 70% of patients who are treated with 40–60mg/day of prednisone for 3–4 months enter remission, compared with 30% of patients treated with placebo (see Figure 11.1) [4, 5]. Ala
Budesonide
Glucocorticoids have pluripotent actions on the immune system, including effects on the synthesis of inflammatory mediators, cellular immunity and neutrophil function [17]. Since the glucocorticoid receptor is widely expressed in tissues, the biological actions of these drugs are not restricted to the immune system. Unpleasant cosmetic effects (acne, moon faces and bruising) and more serious effects, such as metabolic disturbances (hypertension, metabolic bone disease and diabetes) are common and limit the usefulness of these agents [18]. An ideal glucocorticoid should retain the efficacy of conventional glucocorticoid drugs, while minimizing systemic effects. One possible means of achieving this objective is to specifically target the bowel wall as the therapeutic compartment of interest [19]. The development of budesonide as a treatment for active CD is an example of this approach.
Budesonide is a novel glucocorticoid with a potency approximately five times that of prednisone. The systemic effects of budesonide are reduced in comparison to conventional steroid drugs as a result of extensive first pass metabolism to inactive compounds. Thus, a high local anti-inflammatory effect on mucosal surfaces is possible with low systemic activity [20]. Proof of this concept was first demonstrated in asthma therapy, where topical budesonide was shown to be highly effective, with few or no systemic adverse effects [21]. An oral controlled ileal release formulation of budesonide was developed for the treatment of active CD of the ileum and right colon. A Canadian multicenter dose finding study found that, first, 9 mg/day of budesonide was more effective than a placebo for the induction of remission in patients with moderately active CD (51% vs 20%, p < 0.001 ~ and, second, the proportion of patients experiencing glucocorticoid-related adverse effects with this drug was not greater than with placebo treatment (26% 9mg budesonide vs 26% placebo, p > 0.05) [22]. Alc In a second study, Rutgeerts and colleagues compared 9mg/day of budesonide with a standard prednisolone regimen [23]. Although a favorable trend in response rate was observed in favor of prednisolone therapy, the difference in efficacy between the treatment groups was not large (65% vs 52%, p = 0.12). There were fewer glucocorticoid-related adverse events in patients who received budesonide (budesonide 29%, prednisolone 55%, ARR 26%, NNT = 4, p = 0.003). Alc Finally, as described earlier, Thomsen and colleagues have shown that 9mg/ day of budesonide is more effective than 4g/day of 5-ASA and is equally well tolerated [12]. Data from the studies have been summarized in a Cochrane review by Seow and colleagues, who estimated that budesonide was approximately 15% less effective than conventional glucocorticoid therapy, but was much less likely to cause glucocorticoid-related adverse events [24]. Ala Thus, budesonide is an attractive alternative to 5-ASA or prednisone for induction of remission in patients whose disease is restricted to the appropriate anatomical sites.
Antibiotics
A substantial body of experimental evidence supports the notion that bacteria play an important role in initiating and/or sustaining the pathological inflammatory reaction in the bowel wall [25, 26]. Antibiotics have been used empirically for the treatment of active CD for many years, and review articles and textbooks of medicine commonly advocate their use. However, few good data exist to support this endorsement. The Cooperative Crohn’s Disease Study in Sweden compared 800mg/day of metro-nidazole to 1.5g/day of sulfasalazine in 78 patients with active disease [27]. A 25% response rate for both treatments was shown. Accordingly, it is debatable whether these results are more consistent with an equivalent benefit of metronidazole or the lack of any therapeutic effect for either treatment. The largest trial of metronidazole, carried out by Sutherland and colleagues, randomized patients to receive metronidazole (10 or 20mg/kg per day) or a placebo for 16 weeks (n = 105) [28]. Metronidazole therapy produced a dose-dependent decrease of disease activity (decrease in CDAI: metronidazole 20mg/kg 97, 10mg/kg 60, placebo 1; p = 0.001). However, no difference in remission rate was observed (proportion in remission: placebo 25%, metronidazole 10mg/kg 36%, 20mg/kg 27%). Thus, the controlled data that support the efficacy of metronidazole are not impressive. Ald
More recently the quinolone antibiotic ciprofloxacin has been used in combination with metronidazole. Prantera and colleagues randomized 41 patients to receive combined antibiotics (ciprofloxacin 500 mg twice daily and 250 mg of metronidazole four times daily) or methyl pred-nisolone 0.7–1.0mg/kg for 12 weeks [29]. A statistically significant difference in patients entering remission was not demonstrated (combined antibiotic therapy 10/22 (46%), steroid therapy 12/19 (63%), p > 0.05). The small number of patients in this trial does not permit any definitive conclusion regarding the value of combined antibiotic therapy; however, the 17% difference in remission rates in favor of methyl prednisolone is most consistent with a clinically meaningful treatment advantage in favor of glucocorticoid therapy. Ald
Steinhart et al. conducted a double-blind study of oral ciprofloxacin and metronidazole (both 500 mg twice daily), or placebo for eight weeks in 134 patients with active CD of the ileum, right colon or both [30]. All patients received oral budesonide 9mg once daily. At week 8, 21 patients (33%) assigned to antibiotics were in remission, compared with 25 patients (38%) in the placebo group (p = 0.55; absolute difference –5%, 95% CI -21 to 11%). Ala An interaction (p = 0025) between treatment allocation and disease location on treatment response was identified. Among patients with disease of the colon, 9 of 17 (53%) were in remission after treatment with antibiotics, compared with 4 of 16 (25%) of those who received placebo (p = 0.10). Discontinuation of therapy because of adverse events occurred in 13 of 66 (20%) patients treated with antibiotics, compared with 0 of 68 in the group who received placebo (p < 0.001). In patients with active CD of the ileum, the addition of ciprofloxacin and metronidazole to budesonide was an ineffective intervention.
Although this antibiotic combination may improve outcome when there is involvement of the colon, the evidence supporting this possibility comes only from a post hoc analysis of a small number of patients and was not statistically significant. The section on antituberculous therapy for maintenance describes a trial conducted by Selby and colleagues that may also lend some credibility to the concept that alteration of bacterial flora may yet prove to be helpful for induction of remission [31].
In summary, of the traditional and most widely used interventions glucocorticoids are the most effective treatment for inducing clinical remission of active CD. For those patients whose disease is confined to the terminal ileum and/or right colon, budesonide is an attractive alternative to the conventional glucocorticoids because of the lower incidence of adverse events. Sulfasalazine is modestly effective in patients with mild disease activity. Although the newer 5-ASA compounds and antibiotics are used by many clinicians to treat patients with milder forms of the disease, current data do not provide good evidence for the efficacy of these drugs.
Treatment of therapy-resistant or steroid-dependent patients
Munkholm and colleagues have documented the natural history of an acute exacerbation of CD in a cohort of patients from Copenhagen county [32]. One year after an initial course of treatment a high proportion (56%) of their patients were either therapy resistant (20%) or steroid dependent (36%). This observation has led many clinicians to conclude that earlier and more aggressive treatment with immunosuppressives may be warranted in selected patients.
Conventional immunosuppressive drugs
Three drugs or classes of drugs have been most frequently used: the purine antimetabolites (azathioprine (AZA)/6-mercaptopurine), cyclosporin and methotrexate.
Purine antimetabolites
Until recently the use of the purine antimetabolites for the treatment of refractory patients was not widely accepted, perhaps because of the inconsistent results obtained from the early randomized trials of these drugs. However, more recent studies have for the most part confirmed their efficacy. One of the more important trials was conducted by Candy et al., who randomized 63 patients with active CD to receive a standard tapering induction regimen of pred-nisone over three months and either AZA 2.5mg/kg daily or a placebo for 15 months [33]. Although no early (three months) benefit of AZA was identified with respect to remission rates (CDAI < 150 and no prednisone), the proportion of patients who remained in remission over the entire follow-up time was greater in the AZA group (42% vs 7%, ARR 35%, NNT = 3; p = 0.001). Alc This result is consistent with observational data that suggest that the purine anti-metabolites require a minimum of three months to show a treatment effect. In an attempt to overcome this theoretical limitation Sandborn et al. did a small, uncontrolled study in which patients with active CD received an intravenous 1800 mg loading dose of AZA [34]. This strategy rapidly achieved stable erythrocyte concentrations of the thiol metabolites, which are believed to be responsible for the immunosuppressive effects of AZA. Despite this promising finding, a subsequent RCT which evaluated 96 patients showed equally low (eight week) remission rates in patients who received either loading or conventional AZA regimens (25% vs 24%), in spite of achieving steady state nucleotide levels by week 2 [35]. Ald Furthermore, the proportion of patients entering remission did not increase after eight weeks of treatment. It should be noted that all patients in this study received oral AZA in a dose of 2 mg/ kg and that the proportion of these patients who entered remission and withdrew completely from steroids was only 24%, a figure roughly comparable with the expected response to a placebo in many induction of remission studies. The data are consistent with a slow onset of effect for the purine antimetabolites.
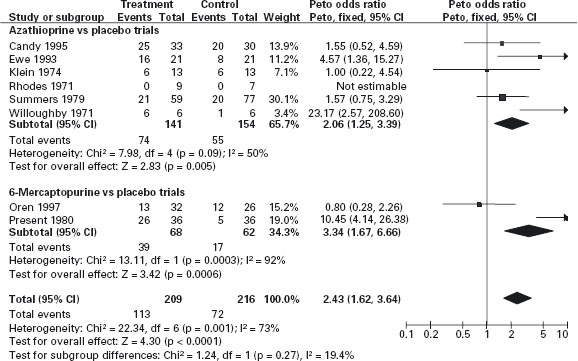
The data from the RCTs which have evaluated the purine antimetabolites for the treatment of active CD in adults have been summarized in a meta-analysis in which the pooled ARR for AZA treatment for induction of remission is approximately 20% (NNT = 5) (see Figure 11.2) [36]. Ald In the majority of these studies patients were receiving concomitant corticosteroid therapy. A steroid-sparing effect was also demonstrated in this analysis: the NNT for steroid sparing (the NNT for AZA to permit one additional patient to reduce steroids to < 10mg/day) was estimated to be 3. These results should be interpreted with a degree of caution, since important clinical heterogeneity exists among the studies in their definitions of treatment response, duration and the use of cointerventions. No single, large, well-designed trial that resulted in a clinically and statistically significant benefit compared with placebo exists. Nevertheless, the meta-analysis suggests that some beneficial effect is present on disease activity, and the use of these drugs can be recommended for treatment of patients who fail to respond to steroid therapy or develop steroid dependence.
Figure 11.2 Azathioprine or 6-mercaptopurine for inducing remission in Crohn’s disease. Source: Cochrane Database Syst Rev 2009; 4: CD000545. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Prefontaine E, Macdonald JK, Sutherland LR. Department of Community Health Sciences, University of Calgary, Health Sciences Centre, Calgary, Canada. Update of: Cochrane Database Syst Rev 2000; 2: CD000545.
Cyclosporine
The emergence of this drug as a standard therapy for organ transplantation led to large-scale evaluations for the treatment of chronically active CD. The results of four RCTs (see Figure 11.3 [37]) have shown that the therapeutic index for cyclosporin is low, if there is any efficacy [38–41]. Alc The study of Brynskov et al. [39], which demonstrated only a modest benefit, used a high cyclosporin dose (7.6mg/kg per day), which cannot be recommended for chronic treatment, since the risk of nephrotoxicity is unacceptably high [42]. The three trials that assessed a dose of cyclosporin that is tolerable for long-term treatment (5mg/kg per day) showed no benefit with this drug [38–40]. Thus, cyclosporin is not a practical therapy for long-term management. Although uncontrolled studies have suggested that short-duration, high-dose intravenous therapy may be beneficial in patients with refractory CD, data from controlled trials would be required to support a recommendation for widespread use [43, 44]. These trials are unlikely to be performed given the demonstrated efficacy and safety of other agents.
Methotrexate
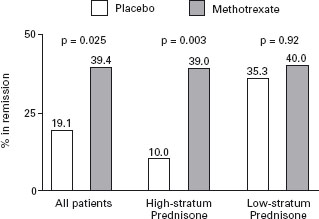
The success of low dose (5–25 mg/weekly) methotrexate as a treatment for rheumatoid arthritis led to its evaluation in patients with chronically active CD. In 1989, Kozarek et al. reported the results of an open study in which two-thirds of patients with steroid refractory disease showed an improvement in symptoms and a concomitant reduction in prednisone requirements [45]. B4 Some patients demonstrated an endoscopic remission. A controlled trial was subsequently conducted in which 141 patients who had failed previous attempts to discontinue prednisone were randomized to receive either methotrexate 25mg/weekly intramuscularly or a placebo for 16 weeks [46]. All of the patients received 20 mg of prednisone per day at the initiation of the trial; a standardized prednisone withdrawal regimen was then used. Patients who responded to therapy discontinued prednisone entirely 12 weeks following randomization. A significant benefit of methotrexate therapy was observed for the primary outcome measure, the proportion of patients who were completely withdrawn from prednisone and in clinical remission as defined by a CDAI score of < 150 points (methotrexate 39%, placebo 19%, ARR 20%, NNT = 5; p = 0.025) (see Figure 11.4). Ala Improvements in the median prednisone dose, Health Related Quality of Life and mean CDAI scores, and concentration of serum acute phase reactants were also associated with methotrexate therapy. In this short-term trial, no serious toxicity was observed, although withdrawals from treatment due to nausea were more common with methotrexate.
Figure 11.3 Point estimates (•) and 95% confidence limits (|–|) of the therapeutic gain (% response cyclosporine–% response placebo) for four RCTs of cyclosporin for Crohn’s disease. Reproduced with permission from Feagan B. Inflamm Bowel Dis 1995; 1: 335–339.
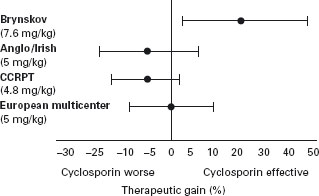
Figure 11.4 Percentages of patients in remission at week 16 according to study group and stratum of daily prednisone dose before entry into the study. The high prednisone stratum was receiving a daily dose of more than 20 mg prednisone, and the low prednisone stratum a daily dose of 20mg or less more than two weeks before randomization. The actual percentages are shown above the bars. P values were derived by the Mantel-Haenszel chi-square test, with adjustment for study center. Reproduced with permission from Feagan BG et al. N EnglJ Med 1995; 332: 292–297.
Novel immunosuppressive drugs
New knowledge of the human immune system and the growth of the biotechnology industry have combined to yield an abundance of new treatments for chronic inflammatory diseases. The development of anti-TNF alpha (inf-liximab, adalimumab, certolizumab) and anti-integrin antibodies (natalizumab) as therapies for CD are examples of the development of this new technology.
Anti TNF alpha antibodies
Tumor necrosis factorα (TNF) is a proinflammatory cytokine which plays an important part in the pathophysi-ology of CD [47]. Following the successful treatment of a young woman with a chimeric anti-TNF-α antibody by investigators in Amsterdam, a series of controlled studies were initiated with several different chimeric or humanized monoclonal antibodies [48].
Infliximab Targan and colleagues carried out a multi-center dose finding study that evaluated 108 patients whose disease was refractory to other forms of treatment [49]. Patients with moderately severe disease received one of three doses of infliximab (5,10, 20mg/kg) or a placebo administered as a single intravenous infusion. Patients continued to receive other treatments at a fixed dose. The primary endpoint of the study was the occurrence of a clinical response as defined by a decrement of 70 points in the CDAI score from the baseline value. No dose-response relationship was identified; 81.5% of infliximab-treated patients responded compared with 16.7% of those who received the placebo (ARR 65%, NNT = 2; p < 0.001). Ala Minor allergic reactions to the antibody occurred infrequently, but clinically significant adverse effects were not encountered in this short-term study.
In a second pivotal trial Present and colleagues evaluated the efficacy of infliximab for the treatment of patients with fistulizing CD (no previous controlled trials had evaluated this population of patients) [50]. The patients studied had active, fistulizing disease for a minimum of three months prior to randomization. Concomitant treatment with steroids, 6-mercaptopurine or AZA, and antibiotics was permitted although the dose of these cointerventions was maintained at a stable level throughout the trial. The primary measure of response was a 50% reduction in the number of open fistulae. Ninety-four patients received three intravenous infusions of either a placebo or one of two dose regimens of antibody (5 or 10mg/kg) during a total of 18 weeks of follow-up. Patients treated with infliximab were significantly more likely to respond (61.9% vs 25.8%, ARR 36.1%, NNT = 3; p = 0.002). Ala The response to treatment was rapid and in many cases dramatic. Again, no dose-response relationship was identifiable.
Adalimumab Adalimumab was developed as a human recombinant immunoglobulin G1 anti-TNF monoclonal antibody for subcutaneous administration. In the CLASSIC-1 trial 299 patients with moderate to severe CD naive to anti-TNF therapy were randomized to receive subcutaneous injections at weeks 0 and 2 of adalimumab 40 mg/20 mg, 80 mg/40 mg, or 160 mg/80 mg, or placebo [51 ]. The optimal induction dosing regimen for adalimumab in this study was 160 mg at week 0 followed by 80 mg at week 2, which produced clinical remission in 36% of patients compared to the rate of 12% observed in the placebo group. (NNT = 4, p = 0.001). Adalimumab was well tolerated.
Certolizumab Certolizumab pegol is a pegylated humanized Fab’ fragment that binds tumor necrosis factor alpha. In the PRECISE-1 study 662 adults with moderate to severe Crohn’s disease were randomly assigned to receive either 400 mg of certolizumab pegol or placebo subcutaneously at weeks 0, 2 and 4 and then every four weeks [52]. Response rates at week 6 were 35% in the certolizumab group and 27% in the placebo group (p = 0.02), although remission rates were not significantly different in the two groups. A1a Serious adverse events were reported in 10% of patients in the certolizumab group and 7% of those in the placebo group. Post hoc analysis of the health-related, quality of life data (self-administered IBDQ questionnaire) from 290 patients showed improvement in emotional well-being and systematic symptoms in certolizumab treated patients at all intervals up to 12 weeks.
Natalizumab
Natalizumab is a recombinant, humanized, monoclonal antibody against the alpha4 integrin. A systematic Cochrane review has summarized the results of pooled data from four randomized trials that investigated the ability of natalizumab, in a variety of dose regimens to induce remission in a total of 1641 patients with moderately to severely active disease [53–57]. Natalizumab (300 mg or 3 to 4mg/ kg) is effective for induction of clinical response and remission in patients with moderately to severely active disease. This benefit was statistically significant for one, two and three infusion treatments, although there was a trend toward increased benefit with additional infusions of natalizumab. The NNT for induction of remission or clinical response varied according to the infusion regimen and outcome and ranged from approximately 4 to 10. For example, pooled data from three studies that included 2456 patients showed that the number of patients required to be treated with a single infusion of natalizumab to produce clinical response at four weeks was eight. Subgroup analysis demonstrated significantly greater clinical response and remission rates for natalizumab compared with placebo in patients in whom active disease was characterized by elevated C-reactive protein levels and active disease despite the use of immunosuppressants or prior anti-tumor necrosis factor therapy.
There were no statistically significant differences between natalizumab and placebo treated patients in the proportions of patients who withdrew due to adverse events or those who experienced serious adverse events. However, the included trials lacked adequate power to detect serious adverse effects that occur infrequently. When two patients with multiple sclerosis treated with natalizumab in combination with interferon beta-1a, and one patient with Crohn’s disease treated with natalizumab in combination with azathioprine developed progressive multifocal leu-koencephalopathy (PML) resulting in two patient deaths, a considerable degree of alarm arose about the safety of this preparation [58–60]. A retrospective investigation was conducted to assess the risk of PML in natalizumab treated patients and no new cases were identified. The incidence of PML has been estimated to be 1 case per 1000 patients (95% CI: 0.2–2.8 per 1000) in this population who received a mean of 17.9 monthly doses of natalizumab [61]. Subsequently, the FDA (US) has approved the use of this antibody for induction of remission in Crohn’s disease.
Combination therapy with anti-TNF alpha antibodies and methotrexate or azathioprine for induction and maintenance of remission
Two important randomized trials have been performed to compare the effectiveness of infliximab alone or in combination with immunomodulator therapy (azathioprine or methotrexate) for induction and maintenance of remission.
In the SONIC trial 508 patients who were immunosup-pressant and anti-TNF naive, and whose mean disease duration was approximately two years, were randomized to receive infliximab plus a placebo (IFX + Pl), azathioprine plus a placebo (AZA + Pl), or infliximab plus azathioprine (IFX + AZA) [62]. The primary endpoint of steroid-free clinical remission at 26 weeks was achieved as follows: IFX + Pl 44.4, AZA + Pl 30.6, and IFX + AZA 56.8 (p < 0.05 for all comparisons). The predefined secondary endpoint of complete mucosal healing was also observed significantly more frequently in infliximab than in azathioprine treated patients. The SONIC trial clearly showed benefit from combined anti-TNF alpha and antimetabolite therapy over monotherapies with these agents.
In the COMMIT study 126 patients with active disease (mean CDAI 208) requiring steroid therapy were randomized to receive standard infliximab induction and maintenance therapy (IFX 5mg/kg weeks 1, 3 and 7 and q 8 weeks ) with either a placebo (IFX + Pl) or methotrexate 25 mg subcutaneously weekly (IFX + MTX) [63]. Prednisone therapy was tapered after the first week to be discontinued by week 14. The combination of IFX and MTX, although safe, was no more effective than IFX alone. Treatment failure rates (percent) at 50 weeks were virtually identical: IFX + Pl 29.8, IFX + MTX 30.6, p = 0.63). This study showed that infliximab combined with initial prednisone therapy can result in very high steroid-free clinical remission rates at one year that are not improved with the addition of methotrexate therapy.
The results of these two trials should not be interpreted as showing that infliximab combined with azathioprine is an effective approach, while the combination with methotrexate is not. No “head-to-head” comparison has been made. The selection of patients and the use of concomitant therapy, particularly prednisone, were quite different in the SONIC and COMMIT trials. All patients in the COMMIT trial received corticosteroids as part of the induction regimen, and remission was achieved in a very high proportion of patients, compared to the rate observed in the SONIC study. It may not be possible to significantly increase the remission rate associated with anti-TNF alpha therapy in combination with steroids by the addition of methotrexate therapy under these circumstances. It is possible that combined therapy with infliximab and methotrexate is more effective than therapy with infliximab alone in other circumstances, for example in the absence of concomitant steroid therapy.
Conventional ‘step-up’ versus ‘top-down’ approach to use of immunosuppressive agents for induction and maintenance of remission
It is clear that anti-TNF alpha antibodies are effective for induction of remission of CD in a patient population refractory to other treatments and that serious short-term toxicity is uncommon. However, potential safety concerns include the formation of autoantibodies, the risk of infusion reactions with re-treatment, and a possible increased risk of lymphoproliferative disease. Therefore, the conventional approach to induction of remission in patients with active Crohn’s disease has been to employ initial therapy with corticosteroids, adding immunosuppressive medications when patients become resistant to or dependent on steroid therapy. D’Haens et al.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







