Some of the factors that contribute to the high prevalence of CAD in patients with ESKD include advancing age and diabetes. The average age of dialysis patients has increased steadily over the past two decades, such that currently their median age is 65 years (1). This older age group has the highest rate of cardiovascular-related deaths in the ESKD population (15). At the same time, even young HD patients have a high prevalence of CVD death: Among ESKD subjects aged 20 to 44 years, the incidence of CVD death is roughly 40 per 1,000 patient-years (15,16). Interestingly, the percentage of deaths that are cardiovascular in cause is similar in all age groups with ESKD (16), leading some investigators to suggest that atherosclerosis may be accelerated in this patient population (17). Also, diabetes mellitus is the leading cause of ESKD in the United States (1). CVD accounts for almost 75% of deaths of patients with diabetes, almost entirely as a result of CAD (18). In a recent study of new diabetic dialysis patients without cardiac symptoms or a known cardiac history, significant CAD was present in 83% (9). In many patients with diabetes mellitus and resultant ESKD, atherosclerosis is well established by the time dialysis is initiated so that many of them manifest ischemic heart disease (IHD), peripheral vascular disease, ischemic bowel disease, or cerebrovascular events shortly after dialysis is begun (19). Not surprisingly, therefore, diabetic patients with ESKD have an extremely high risk for cardiac death.
 PATHOGENESIS OF ATHEROSCLEROSIS IN PATIENTS WITH END-STAGE KIDNEY DISEASE
PATHOGENESIS OF ATHEROSCLEROSIS IN PATIENTS WITH END-STAGE KIDNEY DISEASE
The hypothesis that ESKD, in some way, might cause accelerated atherosclerosis was proposed more than 30 years ago by Lindner et al. (17) who reported a high incidence of MI in the Seattle dialysis population. Of their 39 subjects undergoing HD for an average of 6.5 years, 23 (59%) died, 14 of which were attributed to complications of atherosclerosis (MI in 8, cerebrovascular accident in 3, and refractory congestive heart failure in 3). The incidence of these complications was several times higher than that noted in normal and hypertensive patients of similar age without ESKD. Subsequent postmortem (20) and angiographic (21,22) studies confirmed that the prevalence of atherosclerotic CAD is increased in dialysis patients when compared with age-matched individuals with normal renal function. For example, in a postmortem examination of dialysis patients, Ansari et al. (20) found >50% luminal diameter narrowing of at least one epicardial coronary artery in 60% and at least some degree of atherosclerotic CAD in 86%.
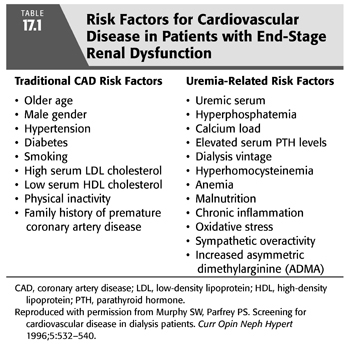
Subsequent studies have shown that the high prevalence of atherosclerosis among patients on maintenance dialysis is the result of the high prevalence of both “traditional” and uremia-related risk factors (TABLE 17.1). Whether maintenance HD in itself promotes progression of atherosclerosis (due, e.g., to exposure to bioincompatible dialysis membranes and/or contaminated dialysis fluids) remains unclear. Long-term studies have shown no correlation between the duration of dialysis and the occurrence of cardiac events, a relation that might be expected if, in fact, dialysis itself promotes atherogenesis (23,24). Dialysis patients have a high prevalence of many of the Framingham risk factors for CAD. A cross-sectional comparison of 1,041 dialysis patients with the general population (via the National Health and Nutrition Examination Survey database) found a high prevalence of hypertension (96%), limited physical activity (80%), diabetes mellitus (54%), hypertriglyceridemia (36%), and electrocardiographic evidence of left ventricular hypertrophy (LVH) (22%) in those with ESKD (25). Even after adjusting for race, gender, and atherosclerotic vascular disease, these factors remained more common in ESKD patients (25). Apart from the increased prevalence of these “traditional” risk factors in patients with CKD (26,27), several uremia-related risk factors are thought to contribute to CAD in these patients (TABLE 17.1), including hyperphosphatemia, oxidative stress, inflammation, and hyperhomocysteinemia. Finally, newly discovered risk factors for atherosclerosis in patients with ESKD may contribute, such as the accumulation of the endogenous inhibitor of nitric oxide (NO) synthase, asymmetric dimethylarginine (ADMA), which results in reduced NO synthesis (28,29).
An increased prevalence of atherosclerotic CAD is progressive over a range of reduced glomerular filtration rates (GFRs) even before the initiation of dialysis (30–33). In a landmark study of >1 million subjects, Go et al. (30) showed that the age-adjusted rate of cardiovascular events (i.e., MI, stroke, heart failure, and peripheral arterial disease) increased markedly below an estimated GFR of 60 mL/min/1.73 m2. After adjustment for a variety of potentially confounding variables, a lower estimated GFR persisted as a graded, independent predictor of adverse outcomes, and this association was robust in those with or without diabetes. CKD patients with known CAD are at the highest risk for subsequent cardiovascular complications and death, with nearly 46% of subjects with an estimated GFR <45 mL/min/1.73 m2 dead within 3 years of follow-up (31). In angiographic studies, patients with CKD were more likely to have severe three-vessel disease and left main disease as estimated GFR decreases (34,35). These findings may explain the high early risk of acute MI after the initiation of dialysis; in one study, 29% of infarctions occur within 1 year and 52% within 2 years of dialysis initiation (10).
 RISK FACTORS FOR ATHEROSCLEROSIS IN PATIENTS WITH END-STAGE KIDNEY DISEASE
RISK FACTORS FOR ATHEROSCLEROSIS IN PATIENTS WITH END-STAGE KIDNEY DISEASE
The Role of Traditional Risk Factors for Coronary Artery Disease
The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study reported a prevalence of traditional risk factors for atherosclerotic CVD in incident dialysis patients (25). This population, which was more likely to be older, African American, and male than the general United States adult population, had a high prevalence of hypertension (96%), low physical activity (80%), diabetes (54%), hypertriglyceridemia (36%), low high-density lipoprotein (HDL) cholesterol (33%), and LVH by electrocardiogram (ECG) criteria (22%). A high proportion of dialysis patients have multiple traditional risk factors such as older age, hypertension, and diabetes (36). Also, smoking seems to be more prevalent in dialysis patients with CAD than those without CAD (37,38).
Hypertension
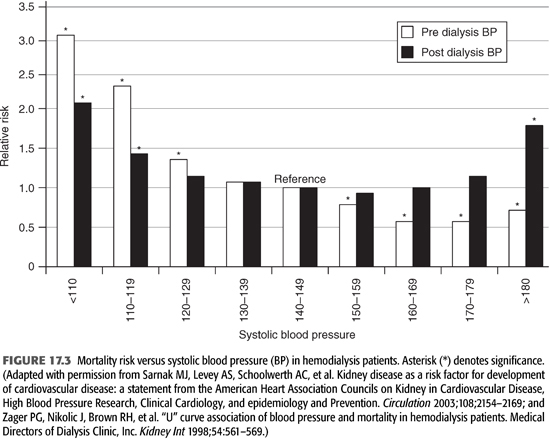
Hypertension, defined as 1-week average predialysis systolic blood pressure (BP) measurements >150 mm Hg or diastolic BP >85 mm Hg or the use of antihypertensive medications, was documented in 86% of clinically stable adult HD patients (39). Among HD patients who were hypertensive, 58% were treated but had uncontrolled BP and only 30% had controlled BP (40,41). The independent determinants of poor control of BP were the use of antihypertensive drugs and an expanded extracellular volume state (42). It has been suggested that hypertension may be better controlled in peritoneal dialysis patients than in those on HD (43–45). However, 44-hour ambulatory BP monitoring showed no differences in daytime and nighttime BP (46). Hypertension is believed to be the predominant risk factor for atherosclerosis in this patient population (47–50). Hypertension in most dialysis patients is systolic (due to arterial stiffness), although an increased pulse pressure (also a reflection of arterial stiffness) is common in these subjects as well. Systolic pressure is more strongly associated with an increased risk of cardiovascular death than either pulse or diastolic pressures in this patient population (51). Interestingly, a U-shaped relation between systolic pressure and mortality is present in dialysis subjects, in that both lower and higher levels are associated with an increased mortality (FIGURE 17.3) (3,52).
Hypertension is usually present for years during the course of CKD before dialysis is initiated. Despite aggressive dialysis and antihypertensive therapy, BP is poorly controlled in about half of ESKD patients. The long duration of poorly controlled hypertension is an important contributor to the increased risk of CAD (53). The increased tensile and sheer stresses caused by hypertension induce endothelial cell injury and activation. This, in turn, is followed by secretion of vasoactive and growth-regulating factors which can elicit a series of cellular interactions that culminate in the appearance and progression of atherosclerosis (54). Aside from its role as a major risk factor for atherosclerosis, poorly controlled hypertension is associated with an increased likelihood of complex ventricular arrhythmias (55–57), and the hypertension-induced increase in left ventricular mass increases the morbidity and mortality of these arrhythmias, should they occur (57–59). Not surprisingly, a reduction of left ventricular mass (e.g., accomplished through an increase in hematocrit, effective antihypertensive therapy, sympathetic nervous system blockade, or improved control of intravascular volume) is associated with a reduced morbidity and mortality in ESKD patients (60–62). Interestingly, conversion from conventional to nocturnal daily dialysis has been reported to induce regression of LVH (63).
Diabetes Mellitus
Diabetes mellitus affects >11% of the adult population in the United States, and its prevalence continues to increase (64). Moreover, diabetes is associated with increased risk of CVD morbidity and mortality in the general population (65,66). Diabetes mellitus is also the leading cause of ESKD in the United States, accounting for nearly 40% of cases (1). In dialysis patients, the presence of diabetes is an independent risk factor for IHD, heart failure, and all-cause mortality (1,26). The incidence of CAD in diabetic patients with nephropathy is 8 to 15 times higher than in diabetics without nephropathy (49,67). Furthermore, diabetic patients with ESKD have a cardiovascular mortality twice that of age-matched nondiabetic patients. In patients with diabetes mellitus in whom coronary angiography is performed routinely as a prelude to renal transplantation, significant CAD is noted in 25% to 50% (21,22,68). The prevalence of CAD in diabetic patients awaiting renal transplantation is particularly high in those older than 45 years (69). Moreover, these patients have worse long-term outcomes after coronary interventions than nondiabetic patients (70,71).
Although virtually all studies of CAD have defined “significant” coronary atherosclerosis as a luminal diameter narrowing >50% to 70%, the extent of luminal diameter narrowing and the occurrence of acute coronary events (unstable angina pectoris, MI, or sudden cardiac death) are not tightly coupled. Ambrose et al. (72) and Little et al. (73) showed that the extent of atherosclerotic coronary arterial narrowing often does not predict the risk of total or subtotal thrombotic coronary arterial occlusion, in that many acute ischemic events are caused by thrombotic occlusion of a coronary artery without a significant stenosis.
Glycemic control improves survival in diabetic patients on HD (74,75). Two studies in Japanese diabetic dialysis patients reported a higher risk of death in those with worse glycemic control (76,77). Furthermore, even mild degrees of hyperglycemia in nondiabetic dialysis populations are associated with reduced survival (78). In short, effective glycemic control in dialysis patients is an important determinant of survival. The hemoglobin A1c assay (HbA1c) is the gold standard test for monitoring glycemic control in patients with diabetes mellitus. Unfortunately, its measurements may not be as reliable in ESKD subjects as in the general population. HbA1c levels may be falsely elevated or decreased in patients with ESKD. This may be due to a number of factors that contribute to shortened red blood cell survival such as uremia, recent transfusion, iron deficiency, erythropoietin-induced accelerated erythropoiesis, and metabolic acidosis. Moreover, the decreased time for glucose and erythrocytes to interact in patients with ESKD leads to lower HbA1c levels for any given level of glycemia compared to those with normal kidney function (79). In some assays such as agar gel electrophoresis, analytical interference from carbamylated hemoglobin formed in the presence of elevated concentrations of urea may lead to false elevations in the HbA1c level. By contrast, the Boronate-agarose affinity chromatography (80–82) and the thiobarbituric acid method (82,83) are more reliable in measuring HbA1c in patients with ESKD. However, other studies have reported that HbA1c significantly underestimates glycemic control in diabetic dialysis patients, whereas those of glycated albumin more accurately reflect this control (84). As a result, glycated albumin may be a more robust indicator of long-term glycemia than HbA1c in HD patients (84). Recent studies have shown that glycated albumin levels more accurately predict hospitalizations and patient survival in ESKD than HbA1c (85,86).
Hyperlipidemia
Dyslipidemia, defined as increased levels of serum cholesterol and triglycerides, is a well-established traditional risk factor for CAD in the general population and in patients with mild to moderate CKD particularly those with nephrotic-range proteinuria (87). The pathogenic role of serum cholesterol as a risk factor in atherosclerotic CVD was confirmed by several randomized clinical trials which demonstrated that reductions in total and low-density lipoprotein (LDL) cholesterol levels, primarily with statins, is effective in reducing coronary artery events and mortality (88–91). Clinical trials have also shown that lipid-lowering therapy is effective in reducing the risk of atherosclerotic cardiovascular events in the nondialyzed CKD population (92,93). Serum lipid abnormalities are frequent in dialysis patients and are considered a major risk for CAD (87,94). These are characterized most often by elevated serum triglycerides and triglyceride-rich lipoproteins—such as very low-density lipoprotein (VLDL)—as well as low HDL cholesterol (TABLE 17.2) (87,95–99). The prevalence of dyslipidemia (defined as the presence of at least one abnormal lipid variable) in ESKD patients is approximately 67% (96). Peritoneal dialysis appears to be associated with a somewhat more atherogenic lipid profile than HD (96). The cause of kidney disease does not appear to influence the specific lipid abnormalities that are seen. The most common pattern, seen in 50% to 75% of patients on HD, is an elevated serum triglyceride concentration in conjunction with a normal LDL cholesterol concentration (87,95–99). This pattern is caused by diminished removal of triglyceride from the serum due to an acquired deficiency of lipoprotein lipase and hepatic triglyceride lipase. Apoprotein (apo) CII, an activator of lipoprotein lipase, is reduced in ESKD patients, whereas apo CIII, an inhibitor of lipoprotein lipase, is increased (24,97). The serum concentration of HDL is reduced substantially in many subjects with ESKD, probably because of a reduction in its synthesis and turnover (TABLE 17.2) (87,95,98,99). Rapoport et al. (98), for example, reported that dialysis patients had an average serum HDL cholesterol concentration of only 26 mg/dL, substantially lower than the average of 52 mg/dL in normal individuals.
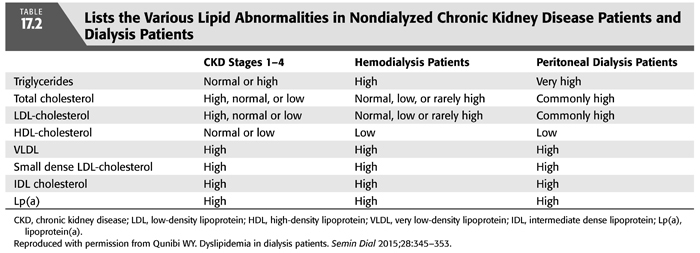
Because hypertriglyceridemia is only weakly associated with CAD in patients with ESKD, it is likely that other more complex lipid abnormalities are important in promoting atherogenesis in these patients (87,97,100–102). Deficiencies of lipoprotein lipase and hepatic triglyceride lipase delay hydrolysis of triglycerides, allowing intestinal and hepatic triglyceride remnants to accumulate, thereby enriching the triglyceride content of VLDL, intermediate-density lipoprotein (IDL), and LDL cholesterol (TABLE 17.2) (102). Apo A-IV and apo B-48, absent in the serum of normal individuals during fasting, are present in patients with ESKD (102), and prolonged exposure of vascular endothelium to these remnant lipoproteins may promote atherogenesis (101,102). The cholesterol content of VLDL is increased and that of HDL is decreased in patients with ESKD (95,97). This cholesterol-enriched form of VLDL, termed β-VLDL (because of its electrophoretic properties), may be more atherogenic than VLDL (TABLE 17.2). In addition, the apo E content of VLDL is increased in patients with ESKD, and this may allow VLDL to interact with LDL at apo B and apo E receptors (97). Finally, lipoprotein (a) [Lp(a)] concentrations are increased in patients with ESKD, independent of the cause of kidney disease (103). All these factors, combined with the decreased antiatherogenic defense mechanisms that accompany a decreased HDL, likely contribute to the development of atherosclerosis.
In patients with early stages of CKD, dyslipidemia contributes significantly to the pathogenesis of atherosclerotic CAD (14,94). This process was thought to be accelerated in HD patients as was initially described by Lindner et al. (17) in 1974. Subsequently, Lowrie and Lew (104) reported a U-shaped relationship between serum total cholesterol level and the risk for all-cause mortality in dialysis patients. Other large observational studies have confirmed the negative relationship between low serum total or LDL cholesterol and CVD in dialysis (105) and nondialyzed CKD patients (106). In one study, serum LDL cholesterol <70 mg/dL was strongly associated with all-cause mortality risk, but black patients displayed a more conventional association between high LDL cholesterol and increased death risk (105). The high risk associated with low cholesterol level was attributed to malnutrition-inflammation complex. To clarify this relationship further, Liu et al. (107) reported that hypercholesterolemia is an independent risk factor for all-cause and CVD mortality in a subgroup of ESKD patients without serologic evidence of inflammation or malnutrition. Given that statins reduce inflammation in addition to cholesterol level, these authors stated that their findings support the use of statins in preventing CVD in dialysis patients.
Oxidative Stress
The association between oxidative stress and atherosclerosis was recognized by Glavind et al. (108) in 1952. Since then, increased production of reactive oxygen species (ROS) in the vascular wall has been recognized as a characteristic feature of atherosclerosis (109). This entity results from an imbalance between reactive oxygen and nitrogen species production and antioxidant defense mechanisms. Oxidative stress with or without endothelial dysfunction occurs commonly in dialysis patients and is thought to contribute to the pathogenesis of CVD (110–112). There has been mounting evidence that ESKD is associated with increased oxidation and impaired antioxidation systems (111–117). Oxidative stress may contribute to atherogenesis by multiple mechanisms that may or may not be linked to LDL oxidation (118) since ROS may mediate pathologic processes in the endothelium, smooth muscle cells, and inflammatory cells. Free oxygen radicals can rapidly react with and inactivate NO and enhance proatherogenic factors, such as leukocyte adherence to the endothelium, impaired vasorelaxation, and platelet aggregation (118).
Exogenous factors may contribute to the increased oxidant activity noted in ESKD patients. The use of incompatible dialysis membranes (cellulosic) may dramatically increase H2O2 release from activated granulocytes (119). This increase in ROS and activated neutrophils in close proximity to endothelial cells may contribute to endothelial cell injury. The chronic, cumulative exposure to cellulosic membranes may augment lipid peroxidation, thereby contributing to the generation of oxidized LDL (117,120). The use of cellulosic membranes may lead to an increased production of ROS by complement-dependent and complement-independent processes (119,121). In addition to the role of exogenous factors, a reduced concentration of endogenous antioxidants, such as vitamins C and E, has been reported in HD patients (122,123).
On the other hand, the antioxidant activity is significantly improved with HD to a level comparable to that of healthy controls, as shown by Nagasi et al. (121). In short, ESKD patients manifest diminished oxygen-scavenging activity, which is improved with dialysis. Some studies have shown a decrease in fatal cardiac events in nonkidney disease patients who consume large amounts of β-carotene or vitamin E (124,125). However, prospective, randomized trials in patients with ESKD have not observed a reduction in the incidence of fatal cardiac events with antioxidant dietary supplementation (126). Nonetheless, vitamin E may slow the progression of atherosclerosis in ESKD patients (127), and endothelium-dependent vasoreactivity may be improved by the combination of antioxidant and lipid-lowering therapy (128,129). In this regard, two randomized trials of antioxidants (N-acetylcysteine and vitamin E) in HD patients reported a decreased risk of cardiovascular events in patients randomized to the antioxidant arm (130,131). Given the small sample size of these studies, further trials are clearly required to confirm their results.
Advanced Glycosylation End Products
Advanced glycosylation end products (AGEs) are formed by a series of complex nonenzymatic reactions between glucose and the amino groups of proteins, peptides, and amino acids. Their serum concentrations are increased up to 10-fold in ESKD patients when compared to healthy subjects. They are not removed by dialysis (132–134). The levels of AGEs in nondiabetic patients undergoing dialysis may be even higher than diabetic nonuremic patients not requiring dialysis. This increase in AGEs levels is due to both an increase in AGE synthesis by oxidative or carbonyl stress and a decrease in AGE excretion by the kidney and the dialyzer (133). Strong evidence exists to suggest a role for AGE compounds in most diabetic vascular complications (134). In this regard, high levels of AGEs can inhibit the production of NO, thereby diminishing vasodilation (134,135). This may result in an increase in BP as well as an increased risk of stroke and ischemic cardiac events.
Advanced glycation end product moieties may also modify lipid biomolecules, particularly LDL cholesterol, which contributes to the development of atherosclerosis (136). On the basis of data from experimental animals, Palinski et al. (137) suggested that AGEs are present in atherosclerotic lesions. Through all these mechanisms, it seems plausible that AGEs may potentiate atherosclerosis in ESKD patients (138). By contributing to the increased oxidative stress that exists in patients with ESKD, AGEs may contribute to the excessive CVD seen in this patient population. In this regard, tissue accumulation of AGEs, as estimated by skin autofluorescence, has been implicated as a risk predictor for cardiovascular mortality in patients with ESKD (139,140). In contrast, the relationship between serum AGEs and mortality in HD patients remains controversial with some studies reporting a strong relationship between serum AGEs levels and survival (141,142), whereas others did not (139,143,144).
Oxidized Low-Density Lipoproteins
As stated previously, dialysis patients are exposed to enhanced oxidative stress initiated by the generation of oxygen free radicals. Oxidation of LDL occurs in vivo and contributes to the development of atherosclerosis by causing endothelial cell injury and apoptosis (145). Cellular lipoxygenase and ROS are believed to induce LDL oxidation (146,147), which leads to a chemical rearrangement of fatty acid structure and subsequent fragmentation, resulting in aldehyde and ketone formation. These alterations favor the recognition of oxidized LDL by monocytes, which act as scavengers in the subendothelial space. Scavenger cells that take up oxidized LDL gradually become cholesterol enriched and form foam cells, the initial “building blocks” of atherosclerosis (148). Leukocyte–endothelial cell interaction further promotes the process of atherosclerosis by potentiating monocyte chemotaxis. In addition, oxidized LDL directly damages endothelial cells (137,149). One intriguing observation is that LDL oxidation can occur in vivo and generate antigenic epitopes (149), leading Salonen et al. (150) to propose that autoantibody formation against oxidized LDL may contribute to the progression of atherosclerosis. Other reactions, such as the carbamylation of LDL, may contribute to altered LDL clearance and influence the scavenger pathway in ESKD patients. An elevated urea nitrogen concentration can trigger condensation of cyanate with lipoprotein lysine residues, leading to reduced clearance and increased pathogenicity (151).
Oxidized LDL is present in chronic dialysis patients, and ESKD patients have an increase in plasma lipid peroxidation products (152–154). In fact, oxidized LDL is more than eightfold higher in chronic dialysis patients when compared with controls (155). In short, ESKD patients on dialysis have increased concentrations of oxidized LDL, which play an important role in accelerated atherogenesis.
Lipoprotein(a)
In CKD, there is accumulation of highly atherogenic lipoproteins such as chylomicron remnants, IDLs, oxidized LDL, small dense-LDL (sd-LDL), and Lp(a) (95). Lp(a) is an LDL-like lipoprotein consisting of apolipoprotein (a) that is covalently bound to an LDL particle. Lp(a) has been linked to atherogenesis in the general population and in ESKD patients (156). In patients with CKD, plasma Lp(a) levels are significantly influenced by the GFR. In patients with large (but not small) apo(a) isoforms, plasma Lp(a) levels begin to rise early in the course of kidney disease (157). This isoform-specific increase in plasma Lp(a) levels has also been observed in HD patients (158). Concomitant malnutrition and inflammation have been associated with high plasma Lp(a) levels in HD patients (159). Its atherogenicity may be related to its binding of apolipoprotein B and subsequent uptake by macrophages, forming foam cells. Lp(a) accumulates in atherosclerotic plaque, after which it undergoes further alterations, including oxidation, which may contribute to its atherogenicity (156). In addition, Lp(a) inhibits plasminogen activation and may stimulate vascular smooth muscle proliferation, each of which may further enhance atherosclerotic plaque formation (160). Lp(a) serum concentrations are typically two to three times higher in ESKD patients than in healthy controls (157,158), resulting largely from a decreased renal catabolism of Lp(a). Certain Lp(a) phenotypes may be particularly atherogenic, for example, the low molecular weight phenotype of Lp(a) may increase the risk of atherosclerosis in ESKD patients. Following successful renal transplantation, the plasma Lp(a) concentration decreases in HD patients with large apo(a) isoforms (161).
Malnutrition–Inflammation Complex Syndrome
Protein-energy malnutrition and inflammation, known as malnutrition–inflammation complex syndrome (MICS), have been implicated as a powerful predictor of death in dialysis patients (162,163). Hypoalbuminemia, a reliable indicator of the presence of MICS, is common in dialysis patients and correlates strongly with a poor outcome, including cardiovascular death (163). In one study, an increase in serum albumin above 3.8 g/dL was associated with improved survival, whereas a falling serum albumin over time correlated with increased cardiovascular death independent of demographic, clinical, or other laboratory variables (164). Malnutrition may contribute to atherosclerosis, in that an inverse relation between low serum albumin and elevated Lp(a) concentrations is present in chronic dialysis patients (159). Whether vascular reactivity is altered by poor nutrition is unknown, but Ritz et al. (165) suggested that malnourished patients have diminished NO production. Although the mechanisms underlying the increased CVD risk of hypoalbuminemia are unclear, it is possible that albumin may act as an oxidative product scavenger so that hypoalbuminemia renders the patient more vulnerable to atherogenesis. In diabetic subjects with ESKD, Koch et al. (166) demonstrated an association between lower skin fold thickness and mortality. Moreover, a low baseline body fat percentage and fat loss over time are independently associated with a higher mortality in dialysis patients even after adjustment for demographics and surrogates of muscle mass and inflammation (167). Also, inflammation is associated with an enhanced cardiovascular risk profile and an increased cardiovascular mortality in HD patients. Inflammation plays a key role in CAD and other manifestations of atherosclerosis (168). A number of plasma markers of inflammation such as high-sensitivity C-reactive protein (hs-CRP), serum amyloid A, and cytokines such as interleukin-6 (IL-6) and soluble intercellular adhesion molecule type 1 have been advocated as potential tools for predicting the risk of CAD events (169). Approximately half of HD patients exhibit an activated acute phase response characterized by increased concentrations of CRP and serum amyloid A (167). In addition, several changes in the atherogenic risk profile in HD patients, such as elevated Lp(a) and fibrinogen levels as well as decreased HDL cholesterol and apo A-I concentrations, are due, at least in part, to an activated acute phase response (170). Finally, the plasma IL-6 concentration, the major mediator of the acute phase response, is elevated in ESKD patients and is considered to be a strong predictor of clinical outcome. Although hypertension, adiposity, insulin resistance, fluid overload, and persistent infections can be associated with elevated IL-6 levels, factors related to the dialysis procedure, such as bioincompatibility of dialyzer membranes and dialysis solutions, also may stimulate its production (171).
Role of Nontraditional Risk Factors for Coronary Artery Disease
Evidence from recent studies suggests that the underlying pathophysiology of CAD is different in patients with CKD when compared to the general population (172). A recent postmortem analysis of CAD showed that the ESKD group had significantly increased coronary arterial medial thickness, higher degree of plaque calcification, and reduced vessel lumen compared with CAD patients and normal renal function (173). Of interest, this study also reported that the frequency of both advanced atherosclerotic lesions and calcified lesions increased as the estimated glomerular filtration rate (eGFR) decreased. In another study that used intravascular ultrasound, which can detect coronary plaque composition, the eGFR was significantly associated with an increase in the percentage of lipid volume and a decrease in the percentage of fibrous volume in coronary lesions with mild to moderate stenosis: a change in composition that may contribute to coronary plaque vulnerability (174). Even after adjustment for coronary risk factors, a low eGFR was independently associated with this shift in coronary plaque composition. Thus, it appears that CKD may independently exacerbate the effects of other conventional CVD risk factors such as hypertension or anemia (175).
Cardiovascular Calcification
The Work Group of the Kidney Disease: Improving Global Outcomes (KDIGO) included cardiovascular calcification in the newly described systemic disorder “chronic kidney disease-mineral and bone disorder (CKD-MBD)” that occurs as a result of CKD (176). The kidney plays a central role in regulating mineral metabolism. Disturbances in mineral metabolism may occur in the course of CKD because of the inability of the failing kidneys to maintain the levels of serum phosphorus and calcium in the normal range. Vascular calcification develops early, and its prevalence increases as the GFR declines such that approximately 80% of incident dialysis patients have evidence of coronary artery calcification (CAC) (177–181) (see Chapter 24 on CKD-MBD).
Vascular calcification results from abnormal deposition of calcium phosphate salts into the vascular wall. This process, which affects coronary arteries and peripheral vessels, may also affect other cardiac structures including the cardiac valves and aorta. Vessels from healthy subjects do not usually calcify even after long-term exposure to supraphysiologic levels of phosphate and/or calcium in vitro, whereas vessels from subjects with CKD and dialysis calcify (182). However, calcification starts as early as CKD stage 2, as shown in an animal model of CKD (183) and accelerates with progressive loss of GFR particularly in patients with ESKD who are receiving dialysis. Vascular calcification in patients with CKD can be atherosclerotic, affecting the intima or medial with calcium phosphate deposition in the media of the vessel wall. It has been argued that medial calcification is a manifestation of accelerated atherosclerosis in patients with CKD (184). However, others argue that atherosclerotic and medial calcification are two distinct entities, with the latter developing more commonly in patients with CKD particularly those with diabetes mellitus (185). Clearly, both forms of calcification can occur concomitantly in patients with CKD.
Vascular calcification in patients with CKD was initially believed to be a passive, physicochemical process in which serum phosphate and calcium are deposited in the arterial wall. However, we now know that vascular calcification is an active, cell-mediated, and highly regulated process similar to bone formation with vascular smooth muscle cells (VSMC) playing a critical role. Just as in bone formation, vascular calcification results from either an imbalance between factors that promote calcification and those that act to inhibit calcification (177,179,186). Incubation of human aortic smooth muscle cells in high phosphate or calcium medium directly induces transformation of VSMC to an osteoblast-like cell (182). It is likely that calcium and phosphate have synergistic procalcification effects (187). Transformed VSMCs lose their contractile function and behave like bone-forming cells that lay down a collagen-1 rich extracellular matrix and form matrix vesicles rich in calcium and phosphate that pinch off from the plasma membrane and interact with the extracellular matrix. These vesicles are capable of initiating mineralization of the vascular wall just as they do so in bone (186). Increased calcium levels promote calcification further by inducing apoptosis of VSMC (182,188,189). Both matrix vesicles and apoptotic bodies serve as nucleation sites for hydroxyapatite, particularly in the presence of low calcification inhibitors such as matrix-Gla protein (MGP) and fetuin-A. Thus, unlike vessels from healthy individuals, vessels from predialysis and, to a much greater extent, dialysis patients have been primed to calcify (182). Susceptibility to calcification in CKD may be related both to a decrease in matrix vesicle calcification inhibitors such as fetuin-A and MGP and to degradation of the extracellular matrix by matrix metalloproteases (MMP), such as MMP-2 and MMP-9, which are upregulated in the arterial wall in CKD patients (186). The latter leads to overexpression of transforming growth factor (TGF)-β, which is thought to be involved in osteoblast differentiation and in enhancing VSMC calcification and arterial stiffness (186).
The cause of accelerated calcification in patients with ESKD is multifactorial and includes both the traditional CVD risk factors and uremia-related risk factors (177) (TABLE 17.1). High serum phosphate, even within the normal range, is associated with a greater risk of coronary calcification and cardiovascular mortality in patients with CKD (190,191). However, recent in vitro studies found calcium to be a stronger procalcification factor than phosphate. In one study in which arterial rings obtained during abdominal surgery from children with normal renal function, those with impaired renal function, and from those on dialysis were exposed to high phosphate or calcium media, calcium-induced calcification more potently than phosphate, but only in rings from patients with CKD or those on dialysis (182). This suggests that VSMC from healthy vessels have effective inhibitory mechanisms that prevent calcification, while those from subjects with CKD, particularly dialysis patients, become susceptible to calcification. Also, both high and low parathyroid hormone levels may increase the risk of vascular calcification (192). Some studies reported an independent association between circulating fibroblast growth factor (FGF)-23 levels with the progression of coronary calcifications (193,194), but more recent data indicate that FGF-23 is not involved in vascular calcification (195). On the other hand, klotho deficiency is a well-recognized factor in this process (196).
One of the factors that was suspected of enhancing vascular calcification in general, and CAC in particular, in dialysis and CKD patients is the calcium load provided from use of calcium-based phosphate binders (CBPBs). While a number of clinical trials (197–199), epidemiologic studies (200,201), and meta-analysis (202) have shown increased risk of all-cause mortality or progression of cardiovascular calcification in CKD patients treated with CBPB, other clinical trials (203,204), epidemiologic studies (205), and meta-analysis (206) did not show a difference between calcium-based and non-CBPBs. In fact, a recent randomized, placebo-controlled pilot clinical trial by Block et al. (207) showed that therapy with both noncalcium and CBPBs resulted in progression of vascular calcification, albeit to a lesser degree in noncalcium phosphate binders. Thus, the effect of calcium load from use of CBPB on progression of cardiovascular calcification, while plausible, remains controversial (208). An important issue that needs to be investigated is whether slowing vascular calcification by any means, including phosphate binders, will translate into improvements in clinical outcomes. Two epidemiologic studies have in fact reported that use of any type of phosphate binder, possibly with the exception of aluminum-based binders, is independently associated with improved survival in dialysis patients compared with no use of binder at all (209,210). A third study in an incident United States Renal Data System (USRDS) cohort that started dialysis in 1996 to 1997, when only CBPBs were used in the United States, found no association between the use of these agents and mortality (211). The KDIGO work group stated that there were inconclusive data to indicate that any one binder has beneficial effects on mortality or other patient-centered outcomes when compared with any other binder (176). However, in patients with CKD stages 3 to 5D, the work group recommends restricting the dose of CBPBs and/or the dose of calcitriol or vitamin D analog in the presence of persistent or recurrent hypercalcemia, arterial calcification, or adynamic bone disease.
The clinical consequences of vascular calcification in patients with CKD are serious. Cardiovascular calcification increases the risk of cardiovascular events including MI, fatal arrhythmia, congestive heart failure, and valvular heart disease (212,213). Measurement of CAC by electron beam computed tomography (EBCT) can help in the evaluation of asymptomatic ESKD patients. Using this technique, several studies have shown a high prevalence of CAC and cardiac valve calcification in HD patients, even those who are young (172,197,198,203,214–218). In the Calcium Acetate Renagel Evaluation (CARE-2) study, we found mitral valve calcification in 55%, aortic valve calcification in 40%, and both in 27% (203). Unfortunately, there is no proven effective treatment for cardiovascular calcification in HD patients. In the ADVANCE (A Randomized Study to Evaluate the Effects of Cinacalcet plus Low-Dose Vitamin D on Vascular Calcification in Subjects with Chronic Kidney Disease Receiving Hemodialysis) Study, subjects with CAC scores ≥30 were randomized to cinacalcet (30 to 180 mg/d) plus low-dose calcitriol, or vitamin D analog (≤2 μg paricalcitol equivalent/dialysis), or flexible vitamin D therapy for 52 weeks (219). The cinacalcet-treated patients had a trend toward lesser progression of calcification of four cardiac structures (coronaries, aorta, mitral, and aortic valves) despite the fact that all subjects received CBPBs. However, the effect was modest and statistically insignificant except for the aortic valve. The clinical significance of these findings is currently unclear but probably at best is likely to be minimal (219). Indeed, the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial tested the hypothesis that cinacalcet, as compared with placebo, would reduce the risk of death and cardiovascular events in dialysis patients with secondary hyperparathyroidism (220). The trial enrolled 3,883 participants from many countries and followed them for up to 5 years. Patients in the cinacalcet group experienced a nonsignificant relative reduction in the primary outcome of only 7% compared to placebo (220). Thus, the trial does not provide clear evidence that treatment of HD patients with cinacalcet provides protection against cardiovascular events.
 CLINICAL PRESENTATION OF CORONARY ARTERY DISEASE IN PATIENTS WITH END-STAGE KIDNEY DISEASE
CLINICAL PRESENTATION OF CORONARY ARTERY DISEASE IN PATIENTS WITH END-STAGE KIDNEY DISEASE
Myocardial ischemia is caused by a relative imbalance of myocardial oxygen supply and demand. Most often, it occurs during transient increases in oxygen demand in the presence of atherosclerotic narrowing of one or more coronary arteries. Less often, it may be caused by (a) a transient primary fall in myocardial oxygen supply, such as that caused by coronary arterial vasospasm, or (b) an excessive augmentation of myocardial oxygen demand without any decrease in supply, such as that which may occur with severe aortic stenosis, a sustained supraventricular or ventricular tachyarrhythmia, or severe sustained systemic arterial hypertension. Myocardial ischemia is usually diagnosed when typical angina pectoris occurs during exertion, emotional excitement, or HD. Physical exertion or emotional excitement cause an increase in the three major determinants of myocardial oxygen demand (heart rate, left ventricular wall tension, and cardiac contractility). HD may induce myocardial ischemia by precipitating hypotension, thereby diminishing coronary arterial blood flow and myocardial oxygen supply, or by provoking tachycardia and/or increased cardiac contractility, thereby increasing myocardial oxygen demand. It is not surprising, therefore, that angina pectoris sometimes occurs during HD.
Chest pain, abnormal findings on physical examination, and electrocardiographic changes, the cardinal features of myocardial ischemia in the general population, may occur in patients with ESKD, but these patients are likely to manifest myocardial ischemia in an atypical fashion. For example, many subjects with ESKD have baseline electrocardiographic ST-T wave abnormalities (221), making the electrocardiographic diagnosis of ischemia difficult or impossible. Not uncommonly, dialysis patients with acute coronary events may be asymptomatic. Such “silent” (painless) ischemia is considered to be present when, in the absence of chest pain, (a) ST segment alterations of ischemia are noted during provocative testing or ambulatory electrocardiographic monitoring, (b) reversible myocardial perfusion defects appear with thallium imaging, or (c) reversible segmental wall motion abnormalities are noted by echocardiography. Although the pathogenesis of such silent ischemia is uncertain, three mechanisms have been proposed. First, some patients may have altered neural pathways, such that they cannot sense the pain of myocardial ischemia. Cardiac transplant recipients, in whom the heart is surgically denervated, and diabetic patients with generalized neuropathy are particularly likely to have such episodes. Second, patients with silent myocardial ischemia may have unusually high thresholds for pain and, therefore, do not sense painful stimuli as other individuals do. Third, differences in the duration and severity of ischemic episodes may explain why some of them occur without pain. Because chest pain is a relatively late manifestation of myocardial ischemia, short-lived episodes may interfere with myocardial relaxation and contraction, induce electrocardiographic and perfusion abnormalities, but resolve before chest pain appears.
The presence of silent myocardial ischemia provides helpful prognostic information. In asymptomatic patients without a cardiac history, silent ischemia identifies those at increased risk of a subsequent cardiac event, such as angina pectoris, MI, or sudden cardiac death. Patients with silent as well as painful ischemia or a previous MI are more likely to have an adverse outcome (i.e., MI, the need for coronary revascularization, or cardiac death) in comparison to those without silent ischemia. Although agents that are useful in treating painful ischemia are also effective in treating silent ischemia, the overall impact of therapy (medical or nonmedical) on prognosis is unknown.
 DIAGNOSIS OF CORONARY ARTERY DISEASE IN END-STAGE KIDNEY DISEASE PATIENTS
DIAGNOSIS OF CORONARY ARTERY DISEASE IN END-STAGE KIDNEY DISEASE PATIENTS
In stable patients with ESKD, the occurrence of angina pectoris, pulmonary edema without major changes in intravascular volume, unexplained hypotension, or a marked change in exercise capacity may trigger an investigation of the presence of CAD. Because most deaths in dialysis patients are caused by CVD, two important assumptions have been used in order to justify screening these patients for CAD. First, dialysis patients have a high frequency of obstructive CAD, even in the absence of angina. In a recently reported cohort of stable asymptomatic HD patients, 41% had obstructive CAD, and almost 30% had stenosis in the proximal segments of at least one coronary artery (222). Second, it is hypothesized that early and aggressive intervention in subjects with asymptomatic CAD could prevent MI and cardiac death.
Despite the high prevalence of CAD in CKD patients, routine screening tests are not currently recommended for most patients in the absence of suggestive clinical symptoms and signs of CAD. Clinical practice guidelines have recommended that coronary angiography or noninvasive testing be performed based on the individual’s estimated risk of CAD (223). Routine coronary angiography in new dialysis patients was proposed as a means of improving the detection and treatment of high-risk patients (222). Among diabetic patients with ESKD who are awaiting transplantation, CAD, symptomatic or asymptomatic, is associated with an increased incidence of allograft failure and mortality (22,47,224,225). Philipson et al. (224) performed coronary angiography in 53 diabetic transplant candidates, detecting significant CAD in 20 (38%). During an average follow-up of 1 year, mortality was 44% in those with and only 5% in those without CAD. Based on data such as these, transplant candidates with angina or evidence of previous MI typically undergo coronary angiography as a part of the pretransplant evaluation, and subsequent transplantation may be denied if CAD is extensive and not amenable to revascularization (224,225).
Patients with ESKD without angina or with atypical symptoms of CAD pose a difficult management problem because many of them, particularly if they are diabetic, have CAD (11). For example, Weinrauch et al. (22) reported that 41% of asymptomatic diabetic patients with ESKD awaiting transplantation had significant CAD, and their 2-year survival was only 22%, compared to 88% in those without CAD. Similar findings were reported by Braun et al. (47), who also noted that depressed left ventricular systolic performance was associated with a particularly guarded cardiovascular outcome (47,68,226). ESKD patients with diabetes, peripheral vascular disease, or previous MI are clearly at higher risk of CAD, major adverse coronary events, or both; as a result, they usually are referred for coronary angiography (222). Such an approach may not be justified in other patients with ESKD. Hence, noninvasive cardiac risk stratification is thought to offer a more appropriate alternative to routine diagnostic catheterization.
Levels of cardiac biomarkers may be chronically elevated in 80% to 90% of asymptomatic patients with ESKD who are receiving maintenance dialysis in the absence of myocardial ischemia, possibly because of myocardial apoptosis or silent microinfarction due to small vessel disease (227). However, serial serum cardiac troponin level elevation indicates acute myocardial damage. Serum concentration of troponin T (cTnT) was consistently found to be associated with all-cause mortality and cardiovascular events in patients with ESKD (228). Thus, elevated serum concentrations of troponin T may offer diagnostic and prognostic information in ESKD patients (228). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines for Cardiovascular Disease in Dialysis endorse the use of the serum troponin concentration for risk stratification in dialysis patients (229).
Serum concentrations of troponin T and troponin I (cTnI) often are elevated in dialysis patients; increased concentrations of these biomarkers are predictive of subsequent death in these individuals (230–232). Measurement of cTnT may have a greater potential for its diagnostics and prognostic capabilities. However, cTnI remains more specific than cTnT in the diagnosis of acute MI (228). Of importance, troponin levels should be obtained just before dialysis, as the dialysis procedure can affect levels (233). Data from Zoccali et al. (234) and deFilippi et al. (35) suggest that ESKD patients with a cTnT >0.07 ng/mL are at a particularly high risk for underlying CAD and its associated adverse events. Other studies have found that cTnT is useful in predicting short-term prognosis in patients with a wide range of renal function (235–237). In addition to the relationship between cTnT and CAD, elevated cTnT levels have been linked to increased left ventricular mass in dialysis patients (238–240).
The increased risk of CAD in patients with ESKD may be mediated, at least in part, through chronic inflammation (241–247). Serum markers of inflammation, such as CRP, brain natriuretic peptide (BNP), and the endogenous inhibitor of NO synthase, ADMA, are elevated in patients with ESKD, and they identify those with a high cardiovascular risk (241). In addition, plasma IL-6 may be useful in predicting cardiovascular mortality in HD patients (244). A study from Pisa, Italy, of 757 HD patients prospectively followed for 30 months found that those with combined high levels of CRP and proinflammatory cytokines had an increased risk of cardiovascular and all-cause mortality (245). In addition, elevated serum concentrations of BNP and its inactive N-terminal fragment, Nt-proBNP, both surrogates of myocardial vulnerability, appear to predict cardiovascular events in this patient population (246,247). Finally, Zoccali et al. (248) suggested that raised plasma concentrations of ADMA in ESKD patients are associated with a 52% increased risk of death and a 34% increased risk of cardiovascular events in dialysis patients.
Screening for Coronary Artery Disease in Candidates for Renal Transplantation
Based on Medicare billing claims, it has been estimated that the cumulative incidence of MI is in the range of 8.7% to 16.7% by 3 years after kidney transplant listing and from 4.7% to 11.1% after kidney transplantation (249,250). Observational studies have also found a high frequency of cardiovascular events in the first months after kidney transplantation (249,251,252). Since most ischemic cardiac events in renal transplant recipients are related to preexisting CAD and occur during the first few months after transplantation (253–256), screening for the presence of CAD is an important component of the pretransplant evaluation. A recent study from Spain that screened 356 patients for CVD before inclusion in the renal transplant waiting list found significant CAD in 38% of patients. Among these, 82% had no cardiac symptoms (257). In asymptomatic patients, screening for CAD should be done if the results are expected to lead to changes in management that improve patients’ outcomes. Moreover, screening should also be cost-effective, and its results may be used to deny transplantation to high-risk patients if they have short life expectancy. However, one study found that in selected high-risk patients, the overall 5-year survival after renal transplantation was superior to the expected 5-year survival with continued dialysis (258).
Evaluation should start with a thorough history and physical examination to identify active cardiac conditions before renal transplantation. In asymptomatic patients who are undergoing pretransplant evaluation, guidelines by the American College of Cardiology/American Heart Association (ACC/AHA) and NKF/KDOQI differ (259). While the NKF/KDOQI recommends noninvasive cardiac stress imaging in patients with diabetes mellitus out of concern for silent (asymptomatic) ischemia, the AHA/ACCF stated that “based on available data, routine noninvasive screening of patients with diabetes mellitus either for peritransplantation cardiac evaluation or for long-term care is not justified by existing evidence.” However, the statement added that noninvasive stress testing may be considered in kidney transplantation candidates with no active cardiac conditions based on the presence of multiple CAD risk factors regardless of functional status (260). Relevant risk factors include diabetes mellitus, prior CVD, over 1 year on dialysis, left ventricle hypertrophy, age >60 years, smoking, hypertension, and dyslipidemia; the specific number of risk factors that should be used to prompt testing remains to be determined, but the committee considers >3 to be reasonable (260). The AHA/ACCF Scientific Statement emphasized the high variability in the sensitivity and specificity of radionuclide stress testing and dobutamine stress echocardiography (DSE) for the detection of significant CAD in patients with ESKD (260). In general, CKD patients may not able to undergo full exercise testing and their ST-segment response may not be specific for myocardial ischemia. Moreover, the sensitivity and specificity of noninvasive cardiac tests in CKD patients is lower than that in the general population (261). Radionuclide perfusion imaging is more sensitive but less specific than stress echocardiography for diagnosis of significant CAD in patients on renal replacement therapy (262).
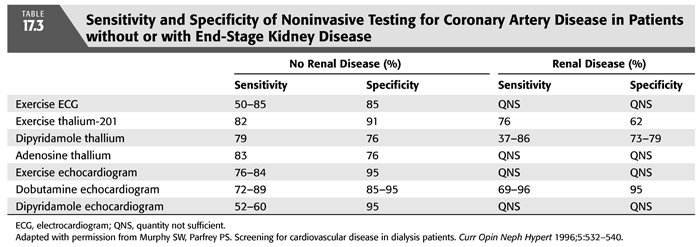
Given that coronary angiography is expensive, invasive, and not risk-free, it is not an ideal screening procedure for all patients with ESKD who are being considered for transplantation. Among its potential deleterious effects, contrast-induced nephrotoxicity may cause further deterioration in patients with some degree of residual renal function. As a result, coronary angiography should be reserved for those who are likely to have significant CAD and may benefit from revascularization (263,264). The reader is directed to FIGURE 17.4 for a suggested approach to the management of patients with ESKD and possible CAD (265). According to the American Society of Transplantation (223), patients with diabetes mellitus, a history of IHD, an abnormal ECG, or age >50 years are at high risk for CAD and should undergo noninvasive stress testing and, if abnormal, then one should proceed to coronary angiography (266). Also, patients with typical angina, previous MI (by history or ECG), or congestive heart failure should be considered high risk, and they should undergo cardiac catheterization to assess left ventricular systolic function and the presence and severity of CAD (267). Other noninvasive studies for the diagnosis of CAD are provided in TABLE 17.3 (268). Patients with congestive heart failure are considered to be high risk because those with CAD and concomitant left ventricular systolic dysfunction have a particularly guarded prognosis and may derive a survival benefit with revascularization (268,269). Even after successful renal transplantation, left ventricular systolic dysfunction is a persistent risk factor for cardiac morbidity and mortality (270).
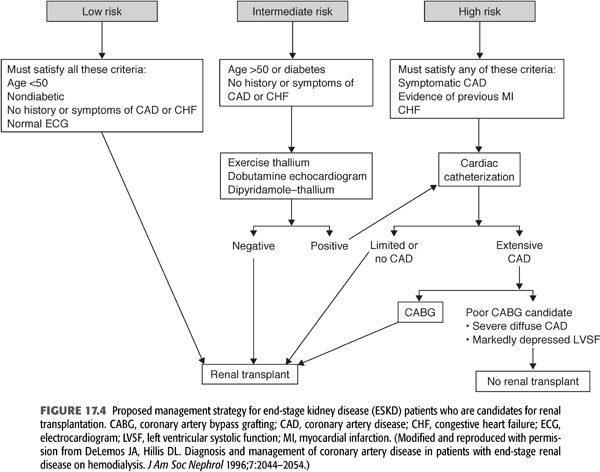
Several investigators have attempted to devise a strategy whereby low-risk patients, who can safely undergo renal transplantation without a preoperative cardiovascular evaluation, can be identified expeditiously, inexpensively, and noninvasively. Le et al. (264) prospectively enrolled 189 consecutive candidates for renal transplantation in a risk stratification program based on the presence of five risk variables: insulin-dependent diabetes mellitus, age >50 years, a history of angina, a history of congestive heart failure, and an abnormal ECG. Patients were considered to be low risk if they had none of these variables and high risk if any was present. Of the 189 subjects, 94 were considered low risk and 95 high risk. Over a follow-up period averaging almost 4 years, cardiac mortality was 17% in the high-risk subjects and only 1% in the low-risk individuals (264).
Other investigators have used a similar approach in diabetic patients with ESKD. In a retrospective study, analysis of the cardiac catheterization data of 97 asymptomatic type 1 and 2 diabetes mellitus kidney and kidney-pancreas transplant candidates revealed that 33% of type 1 and 48% of type 2 diabetic patients had significant CAD (271). By multivariate logistic regression analysis, a body mass index greater than 25, older age, and cigarette smoking were associated with CAD. African American patients, who comprised 30% of the sample, had a 71% lower risk of CAD compared with whites (p = 0.03) (271). Manske et al. (272) retrospectively identified several clinical variables that were associated with CAD in diabetic transplant candidates: age older than 45 years, more than 5 pack-year history of cigarette smoking, more than 25 years of diabetes mellitus, and nonspecific ST-T wave abnormalities on a resting ECG. In a small group of subjects studied prospectively, they showed that these variables provided a sensitivity of 97% and a negative predictive value of 96% for detecting angiographically significant CAD. Furthermore, they concluded that diabetic patients younger than 45 years with none of these variables could safely undergo renal transplantation without a preoperative cardiovascular assessment.
Most renal transplant candidates are at intermediate risk for CAD. This group includes older patients without symptoms of CAD, previous MI, or congestive heart failure, as well as most diabetic patients with no symptoms or atypical symptoms of CAD. In these subjects, noninvasive testing may be particularly useful in the pretransplant cardiac evaluation (273–275). Many patients with ESKD have electrocardiographic abnormalities at rest that make the ECG difficult or impossible to interpret during provocation (often because of LVH). Moreover, other patients, particularly those who are diabetic, fail to attain a sufficient heart rate during exercise to provide a reasonable predictive accuracy (22,134,276). As a result, provocative pharmacologic testing with concomitant cardiac imaging (using nuclear scintigraphy or echocardiography) has been used in an attempt to identify patients with underlying CAD.
Conflicting reports have appeared concerning the utility of thallium imaging with dipyridamole to identify CAD in patients with ESKD awaiting transplantation. On the one hand, Marwick et al. (277) and Boudreau et al. (278) concluded that such imaging was of little use in identifying angiographically significant CAD or in predicting cardiac prognosis in this patient population. Of Marwick and colleagues’ (277) transplant candidates, thallium imaging with dipyridamole offered only fair specificity and poor sensitivity in identifying CAD. Importantly, five of the six individuals who died of cardiac causes over a mean follow-up of 2 years had normal thallium imaging studies. Of the 80 patients reported by Boudreau et al. (278), 36 had negative dipyridamole thallium studies, 6 (17%) of whom had significant angiographic CAD, giving a negative predictive value of only 83% (TABLE 17.3). In contrast, several reports have concluded that thallium imaging with dipyridamole provides an effective noninvasive means of identifying transplant candidates with no chest pain or atypical chest pain who are at increased risk of having a subsequent cardiac event. Camp et al. (274) showed that 6 of 9 patients with dipyridamole-induced reversible perfusion abnormalities had a subsequent cardiovascular event, whereas none of 31 patients without reversible defects had a subsequent event. Other studies (226,279) have supported the contention that thallium imaging with dipyridamole can identify those at increased risk of an adverse cardiovascular outcome. In addition, a normal thallium imaging study appears to predict a very low likelihood of a subsequent cardiovascular event (226,264,274). The reasons that these dipyridamole-thallium data are disparate are not clear but may include differences in study design, definition of end points, interpretation of “positive” thallium images, and patient selection. In addition, most of these studies included only patients referred for noninvasive evaluation, and the differences in referral patterns between centers may explain some of the variability in results.
Two-dimensional echocardiography during the intravenous infusion of dobutamine (so-called dobutamine stress echocardiography) was evaluated in 97 patients (some diabetic, others not) with ESKD awaiting renal transplantation (275). In these patients, this technique had a sensitivity of 95% and a specificity of 86% for predicting cardiovascular complications or death during the subsequent year. Although the positive predictive value was poor (only 14%), the negative predictive value was excellent (97%) (TABLE 17.3). In a more recent study, renal transplant candidates had coronary angiography, DSE, and resting and exercise electrocardiography. Of the 125 patients, 36 (29%) had severe CAD, 55% were on dialysis, and 39% were diabetic. Stepwise logistic regression analysis identified an abnormal resting ECG and a positive stress echocardiogram result as independent predictors of severe CAD (280). Finally, a meta-analysis of 12 observational studies involving 913 patients was performed to determine the prognostic significance of myocardial perfusion studies on future MI and cardiac death in patients with ESKD assessed for kidney or kidney-pancreas transplantation (281). In comparison to patients with normal tests, those with evidence of inducible ischemia (reversible perfusion defects or new wall motion abnormalities) had a sixfold increased risk of MI and an almost fourfold increased risk of cardiac death. In addition, those with noninducible ischemia (fixed perfusion defects or wall motion abnormalities at rest) had a significantly higher risk of cardiac death (RR 4.7). Diabetic patients had a similar pattern of risk, with reversible perfusion defects alone strongly associated with MI (RR 9.3) and both fixed and reversible perfusion defects associated with cardiac death (RR 4.0 to 4.7). In an assessment of symptoms, electrocardiographic findings, thallium dipyridamole scintigraphic results, and resting echocardiographic results in 42 ESKD patients and 42 patients after renal transplantation, the presence of angina offered the best prognostic information (282), whereas the other variables lacked sensitivity and/or specificity in the identification of those with CAD.
In summary, all high-risk transplant candidates (those with symptoms of CAD, previous MI, or congestive heart failure) should undergo coronary angiography before renal transplantation. At the opposite extreme, young, nondiabetic patients without symptoms of CAD or previous MI comprise a low-risk group, and they do not require cardiac evaluation before transplantation. In addition, young diabetic patients, particularly nonsmokers who have had diabetes for less than 25 years and whose ECGs are normal, may not require cardiac evaluation before renal transplantation. Subjects in the intermediate risk group include older patients without symptomatic CAD. In preparation for transplantation, these individuals should undergo noninvasive evaluation (DSE or dipyridamole-thallium imaging), recognizing that neither procedure is perfect. Because most insulin-dependent diabetic patients older than 45 years have underlying CAD, we recommend that these individuals routinely undergo coronary angiography, even in the absence of angina or evidence of previous MI (FIGURE 17.4) (265,269,283).
 MANAGEMENT OF CORONARY ARTERY DISEASE IN PATIENTS WITH END-STAGE RENAL DISEASE
MANAGEMENT OF CORONARY ARTERY DISEASE IN PATIENTS WITH END-STAGE RENAL DISEASE
Medical Management
Evidence-based recommendations for the medical management of CAD in patients with ESKD are hampered by the lack of data from randomized controlled trials because the studies which demonstrated that aspirin, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, and lipid-lowering agents are efficacious in patients with CAD excluded patients with ESKD (284). Because of concern that these agents have limited efficacy and/or enhanced toxicity in subjects with ESKD, they often are underused in this patient population (12). Given that the etiology of CVD in ESKD is complex and may be different from that in the general population, randomized trials that evaluate the efficacy and safety of these drugs in patients with ESKD are still needed. Until such studies are performed, the medications noted previously should be used in patients with ESKD. In addition, correction of the hemoglobin concentration to 10 to 11 g/dL to improve oxygen-carrying capacity should be pursued with erythropoietin therapy and intravenous iron supplementation (285). Such an improvement in hemoglobin may allow the patient to participate in exercise training, which offers additional benefits (286,287). Adequate BP control is critically important in reducing the magnitude of LVH and myocardial oxygen demand, resulting in reduced angina frequency, ventricular irritability, and perhaps even mortality (287–289). Several studies have shown an independent association between measures of glycemic control and mortality in patients with or without diabetes (290). The role of glycemic control in diabetic ESKD was examined by Shurraw et al. (291) in 1,484 incident HD patients during a follow-up of up to 8 years. The authors found that increased serum glucose and HbA1c levels were not independently and directly associated with mortality and strict glycemic control may not benefit dialysis patients with or without diabetes mellitus (291). Other interventions such as smoking cessation, exercise, dietary salt reduction, weight loss, and control of hypertension should be tried, although clear evidence for these measures in ESKD patients is limited.
Several antianginal medications may be useful in dialysis patients with stable angina. Long-acting nitrates are effective orally or cutaneously in reducing angina frequency and severity, but their influence on survival is unknown. Twice-daily dosing with a nitrate-free period is recommended so that nitrate tolerance is minimized. The use of sublingual nitroglycerin may induce hypotension, particularly if the patient uses it during dialysis. Other drugs that are often prescribed in patients with ESKD and angina include β-blockers, which reduce heart rate, contractility, and left ventricular wall tension, and calcium channel blockers, which induce coronary arterial vasodilation and simultaneously reduce the determinants of myocardial oxygen demand. These agents reduce angina frequency and the frequency of cardiovascular events in patients with painless myocardial ischemia (292). Some of these agents should be given with caution to patients with ESKD because they are excreted unchanged by the kidney. However, since the calcium channel blockers diltiazem, verapamil, and the dihydropyridines are metabolized by the liver, dosing adjustments are unnecessary in patients with ESKD.
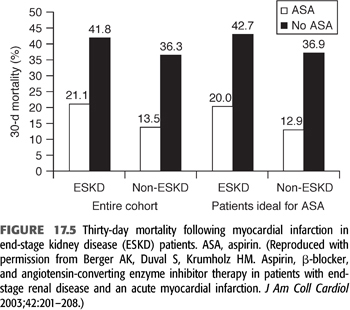
For patients with known CAD with or without ESKD, low-dose aspirin is recommended. Unfortunately, the frequency of aspirin use in dialysis and CKD patients who have survived an MI is low in comparison to those without CKD: In one study, the rate of aspirin use was only 61% in dialysis patients compared to 89% in subjects with normal renal function (293). This reduced frequency of aspirin use is due, at least in part, to safety concerns because aspirin prolongs the bleeding time. Subgroup analyses of randomized trials have demonstrated convincing cardiovascular risk reduction from daily aspirin in individuals with estimated GFR <45 mL/min/1.73m2, including dialysis patients, although the risk of bleeding is higher in these patients (294,295). In survivors of MI, aspirin reduces mortality irrespective of renal function. The efficacy of aspirin after MI was examined in 1,025 patients with ESKD and 145,740 controls (296). The benefit of aspirin treatment on 30-day mortality was similar in the two groups (FIGURE 17.5). In short, the routine use of aspirin after MI in all CKD patients could save one life for every five patients treated (296). To minimize bleeding risks, low-dose aspirin, 81 mg/d, is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


