Chapter 51 Congenital disorders of the pancreas
Surgical considerations
Embryologic Development of the Pancreas
The basis for the understanding of congenital abnormalities of the pancreas is the embryologic development of the organ (see Chapter 1A). The pancreas develops from two buds originating from the endodermal lining of the duodenum. The dorsal bud forms posteriorly within the mesentery, whereas the ventral bud is associated with the hepaticopancreatic duct (Fig. 51.1). During the second month of development, the stomach rotates and the duodenum becomes C-shaped. As part of this process, the ventral pancreatic bud migrates dorsally, coming to lie posteroinferiorly to the dorsal bud, forming what will become the inferior head/uncinate process of the pancreas. In the majority of individuals, the main pancreatic duct (of Wirsung) is formed by the entire ventral duct, and the distal dorsal duct and enters the duodenum at the major papilla. The persistence of the proximal part of the dorsal duct occurs in approximately 25% and results in an accessory duct (of Santorini), which enters the duodenum by the minor papilla (see Fig. 51.1) (Rösch et al, 1976). There is no known pathologic consequence of this normal variation. A failure of fusion of the ductal system occurs in roughly 10% of the normal population (Dawson & Langman, 1961; Millbourn, 1959), resulting in the entire dorsal pancreas—superior head, body, and tail—draining through the minor papilla and ventral pancreas, the inferior head and uncinate process, and finally through the major papilla (Fig. 51.2). This abnormality is termed pancreas divisum and is described in more detail below.
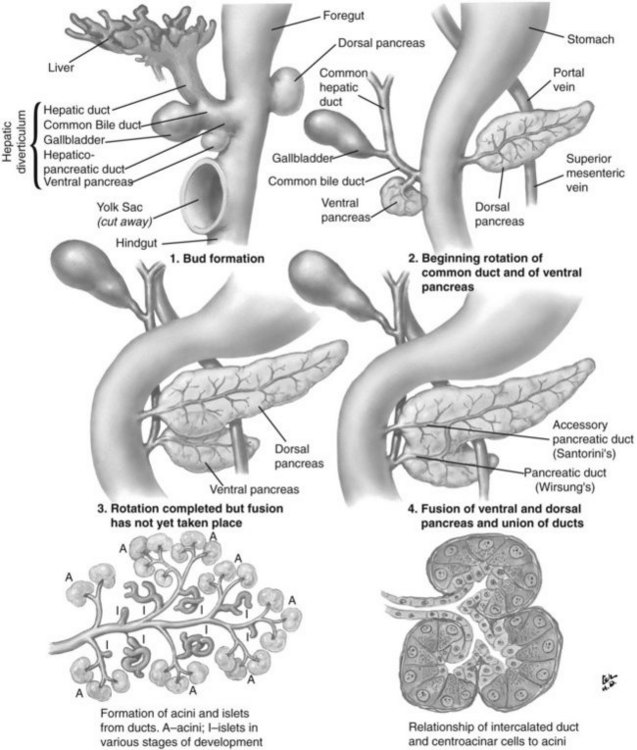
FIGURE 51.1 Embryologic development of the pancreas.
(Netter illustration from http://www.netterimages.com. Copyright Elsevier, Inc. All rights reserved.)
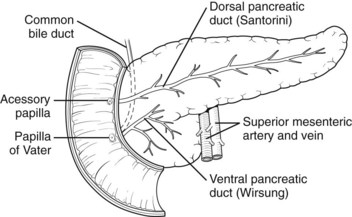
FIGURE 51.2 Pancreas divisum describes the incomplete fusion of the dorsal and ventral pancreatic ducts.
A number of studies published recently have identified mechanisms by which the pancreas is specified from the early endoderm (Kumar & Melton, 2003). Retinoic acid and bone morphogenic peptide both appear to have important roles in defining early endodermal compartments in the embryo (Stafford & Prince, 2002; Tiso et al, 2002). The origins of the signaling mechanisms involved in the specification of the dorsal and ventral pancreas are different. In the dorsal pancreas, signals arising from the notochord (Kim et al, 1997) and dorsal aorta (Lammert et al, 2001) are required; in the ventral pancreas, the lateral plate mesoderm is important (Kumar et al, 2003). The specific identity of these signals has not yet been established, although the Hedgehog family of signaling molecules appears to be significant (Kim & MacDonald, 2002).
Pancreas Divisum
Pancreas divisum (PD) results from incomplete fusion of the dorsal and ventral pancreatic ducts toward the end of the second month of embryogenesis. The distal dorsal pancreatic duct typically fuses with the ventral pancreatic duct to drain the entire pancreas into the duodenum via the major papilla (see Chapter 1B). The proximal dorsal duct can persist as an accessory pancreatic duct and may drain via the minor papilla. Complete PD exists when there is no communication between the dorsal and ventral systems and the majority of the pancreas drains via the dorsal duct through the minor papilla (see Fig. 51.2). Variations exist where a small branch may connect the two ducts, termed incomplete pancreas divisum.
Recent investigations have suggested that PD may be explained by distinct patterns of incomplete fusion of branches of the dorsal and ventral pancreatic ducts (Kamisawa et al, 2007a), confirming theories first proposed in early anatomic studies (Dawson & Langman, 1961). The first description of PD is from 1865, attributed to Josef Hyrtl (1810-1894), professor of anatomy at the Universities of Prague and Vienna. However, as discussed by Stern (1986), a number of anatomists were aware of it much earlier than this, including Regnier de Graaf, who described the finding in 1664.
In postmortem studies performed throughout the twentieth century, the prevalence of PD is reported to be approximately 8% (range, 4% to 14.5%) (Baldwin, 1911; Berman et al, 1960; Birnstingl, 1959; Cameron, 1924; Charpy, 1898; Dawson & Langman, 1961; Fogel et al, 2007; Clairmont & Hadjipetros, 1920; Hand, 1963; Helly, 1898; Hjorth, 1947; Keyl, 1925: Kleitsch, 1955; Mehnen, 1938; Millbourn, 1959; Naatanen, 1941; Opie, 1903; Rienhoff, 1945; Schirmer, 1893; Schmieden & Sebening, 1927; Schwarz, 1926; Sigfusson et al, 1983; Smanio, 1969; Stimec et al, 1996). With the development of endoscopic retrograde pancreatography (ERCP) in the late 1960s, however, the considerable congenital variation in the ductal system of the pancreas became more widely appreciated (Cotton, 1972; McCune et al, 1968). The prevalence of PD is lower in published ERCP series than in anatomic series for reasons that are not clear, although it may be due to referral bias, difficulty in the interpretation of pancreatograms, or inability to cannulate the minor papilla. The reported prevalence is particularly low in Japanese series (0.3% to 0.6%) compared with Western populations (approximately 5%; Cotton, 1980).
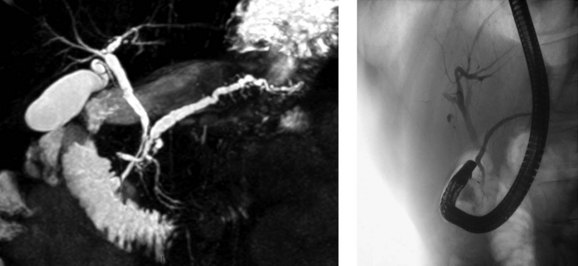
PD is clinically relevant from three main points of view (Fogel et al, 2007). First, the small ventral duct seen in PD must be differentiated from a similar appearance seen in some cases of pancreatic cancer. Second, on cannulating the major papilla at ERCP, only the ventral portion of the pancreas may be visualized in PD, which risks missing important pathology if a pancreatogram is not performed via the minor papilla (see Chapter 18). Third, PD may be associated with disease, such as pancreatitis (see Chapter 53). With regard to the first point, it is important for those performing ERCP to be aware of the anomaly to become proficient in interpreting pancreatograms (Fig. 51.3). Other forms of imaging, such as computed tomography (CT), must be used if there is uncertainty whether a mass lesion is present and causing a ductal abnormality. In relation to the second point, it is important to become proficient at cannulation of the minor papilla, which is considerably more challenging than cannulating the major papilla (see Chapter 18). It commonly sits in a superior, more ventral position. Cannulation may be facilitated by having the duodenoscope in the “long position” and by administration of intravenous secretin (O’Connor & Lehman, 1985). The third point is discussed in detail below.
Possible Association Between Pancreas Divisum and Pancreatitis
The question of whether PD causes recurrent acute pancreatitis, chronic pancreatitis, or pancreas-related pain has been debated for many years (Table 51.1). Those proposing the theory suggest that obstruction occurs at the level of the minor papilla, the caliber of which is too narrow to drain the majority of the pancreas. This is supported by an early study reporting an elevated pressure in the dorsal duct (23.7 ± 1.3 mm Hg) compared with the ventral duct (10.8 ± 1.9 mm Hg) in six patients with PD, whereas pressures were similar when two ducts existed in patients without PD (Staritz & Meyer zum Buschenfelde, 1988). This was contradicted in a subsequent study in which no difference in duct pressures was observed between the major and minor papillae in four patients with recurrent acute pancreatitis and PD (Satterfield et al, 1988). Another early report demonstrated chronic pancreatitis confined to the dorsal pancreas in two pancreatoduodenectomy specimens from patients with PD who had resections for symptom control (Blair et al, 1984).
Difficulty exists in demonstrating an epidemiologic relationship between PD and pancreatitis because early anatomic studies were small and confounded by different definitions, and ERCP series are skewed by selection bias (Blair et al, 1984). In the largest early series of 1850 successful major ampulla cannulations, Rösch and colleagues (1976) identified PD in 63 cases (3.4%). The indications for pancreatography were not given, but pathologic findings were seen in 13 of 63 patients with PD; changes were consistent with pancreatitis in 12 and tumor in one. In the next large series by Gregg (1977), 33 patients with PD were identified among 1100 patients referred primarily for investigation of pancreatic-type pain or pancreatitis. Documented pancreatitis was present in 15 patients, and another 11 had recurrent episodes of pain typical of pancreatitis.
These and similar studies have been used as evidence of a link between PD and pancreatitis, but the absence of a suitable control group makes the assertion weak. Mitchell and colleagues (1979) identified this problem and performed a retrospective analysis of patients who had undergone ERCP and observed that 21 (4.7%) of 449 patients had PD, whereas 4 (3.3%) of 120 patients with pancreatitis defined by clinical and/or ERCP criteria had PD. Thus in this series the prevalence of PD in patients with pancreatitis was the same as the prevalence of PD in the series as a whole. This has been supported by the largest published ERCP series of 304 patients, in which the dorsal duct was visualized in 97 patients (Delhaye et al, 1985). The frequency of PD was similar in patients with acute or chronic pancreatitis (6.9%) as in all patients in the series undergoing ERCP (5.7%).
Contrary to these findings, Cotton reported that in patients with primary biliary disease who had an incidental pancreatogram at the time of endoscopic cholangiogram, the prevalence of PD was 3.6% (Cotton, 1980). This was compared with a prevalence of 16.4% in those with chronic or recurrent acute pancreatitis. In 83 patients with idiopathic pancreatitis, recurrent pancreatitis with no clear etiology such as gallstones, alcohol, or trauma—the prevalence of PD was 25.6%.
Imaging in Pancreas Divisum
One of the difficulties in relating PD to pancreatitis is the inconsistency in making the diagnosis. ERCP studies of patients without pancreatitis consistently report a lower prevalence of PD when compared with MRCP or postmortem studies (Table 51.2). The use of secretin during MRCP (S-MRCP) improves the detection rate of PD (Manfredi et al, 2000), yet it is still reported to be missed on S-MRCP, possibly because of suboptimal magnetic resonance techniques or inexperience in those reporting the MRCP (see Chapter 17; Carnes et al, 2008). Endoscopic ultrasound (EUS) as an alternative imaging modality has gained in popularity and is reported to be useful in the investigation of patients with acute recurrent pancreatitis of unknown etiology (see Chapter 14; Petrone et al, 2008). A recent study reported the use of portal venous phase 64 multirow detector CT. Five of 93 patients had PD diagnosed on MRCP or ERCP. Of these, one observer detected three cases and a second observer found four cases by reviewing the MDCT images (Anderson & Soto, 2009). ERCP features of the minor papilla that suggest the presence of PD include an enlarged papilla or open orifice and are believed to moderately predict the presence of PD; however, a significant number of patients with PD do not have these features (Alazmi et al, 2007; Lawrence et al, 2009).
Table 51.2 Prevalence of PD in Patients Without Pancreatitis by Method of Investigation or Imaging Modality
| Investigation Type | N | % Prevalence of PD (95% CI) |
|---|---|---|
| Postmortem | 2895 | 7.8 (6.8-8.8) |
| MRCP | 505 | 9.3 (6.8-11.8) |
| S-MRCP | 156 | 17.9 (11.9-24.0) |
| ERCP | 16,078 | 4.1 (3.8-4.4) |
PD, Pancreatis divisum; MRCP, magnetic resonance cholangiopancreatography; S-MRCP, secretin-enhanced MRCP; ERCP, Endoscopic retrograde cholangiopancreatography; CI, confidence interval
From Fogel EL, et al, 2007: Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 34:21-45.
Therapy to the Minor Papilla in Those with Pancreas Divisum and Pancreatitis
If PD is associated with obstruction at the minor papilla, which in turn contributes to the occurrence of pancreatitis and pancreatic pain, then it follows that surgical intervention to relieve this obstruction would be beneficial. As has been discussed, significant controversy exists as to whether this assumption is correct or not. A number of studies have been performed examining whether therapy to the minor papilla is beneficial in patients with acute recurrent pancreatitis, chronic pancreatitis, or pancreas-related pain; however, little in the way of randomized controlled data has been generated (Table 51.3; see Chapter 53, Chapter 55A, Chapter 55B ). Interventions examined include endoscopic dilation, papillotomy, and sphincterotomy of the minor papilla, with or without stent placement, and surgical sphincteroplasty.
The first minor papilla endoscopic sphincterotomy was described by Cotton in 1978, and the majority of publications since have been small case series with short follow-up times. Lehman and colleagues (1993) described the effects of minor papilla sphincterotomy in 52 PD patients with chronic pancreatic pain (n = 24), recurrent acute pancreatitis (n = 17), or chronic pancreatitis (n = 11) with longstanding symptoms refractory to conservative management. Minor papilla sphincterotomy was performed with a needle knife over a previously placed dorsal pancreatic duct stent, with a mean follow-up of 1.7 years. When compared with the chronic pain and chronic pancreatitis groups, patients with recurrent acute pancreatitis had a significant reduction in mean symptom score (recurrent acute pancreatitis, 76.5%; chronic pancreatitis, 27.3%; chronic pain, 26.1%) and inpatient hospital stay. This reflects other published results that consistently show less benefit after minor papilla therapy in those with chronic pancreatitis or chronic pain compared with those with recurrent acute pancreatitis. Complications were seen in 15%, and one patient died of a pancreatic abscess after a failed cannulation. Of concern, 50% of patients evaluated at the time of stent removal had stent-induced dorsal duct changes.
In a prospective, randomized, controlled trial published by Lans and colleagues (1992), 19 patients with PD and recurrent acute pancreatitis—two or more episodes of abdominal pain associated with a rise in amylase greater than twice the upper limit of normal—and no other identified etiology were recruited. Ten patients were randomized to stent placement, and nine were randomized to no treatment, with follow-up for 1 year. In the stent group, no patients subsequently came to the hospital with pain, but five patients in the control group were admitted, and two more came in with pain. Furthermore, nine patients in the stent group rated their pain improved by 50% or more, but only one patient in the control group reported a similar improvement.
It has been pointed out that a significant period of time can exist between attacks of pancreatitis in this patient group, and this study has since been criticized on the basis of the short duration of follow-up. Sherman and colleagues (1994) have also published randomized data in abstract form in which patients with chronic abdominal pain thought to be pancreatic in origin and PD were randomized to minor papilla sphincterotomy (n = 16) or no intervention (n = 17). Mean follow-up time in the treated and untreated groups was 2.1 and 1.2 years, respectively. Although an improvement in pain was seen in 43.8% of treated patients compared with 23.5% in the control group, this trend did not reach statistical significance.
Borak and colleagues (2009) published the long-term outcomes after endoscopic minor papilla therapy in 145 patients with PD over a 6-year period. Follow-up data were available for 113 patients (78%), and the median follow-up time was 43 months. The majority of patients had a needle-knife sphincterotomy (82%) and temporary stent placement (90%). Primary success, defined as the patient being better or cured after a single ERCP session, was seen in 53.2% of patients with recurrent acute pancreatitis, 18.2% of those with chronic pancreatitis, and 41.4% of those with chronic/recurrent epigastric pain. Two or more ERCP sessions were required in 41.6%, with success in 71% of patients with recurrent acute pancreatitis, 46% of those with chronic pancreatitis, and 55% of those with chronic, recurrent epigastric pain. In a multivariate analysis that corrected for age, sex, symptom frequency/duration, and length of follow-up, chronic pancreatitis and younger age both independently predicted failure of improvement after treatment. Complications occurred in 13% after ERCP, including mild and moderate pancreatitis, mild bleeding, and anesthetic complications.
Surgical sphincteroplasty to the minor papilla, usually combined with cholecystectomy and major papilla sphincteroplasty, has been used in the treatment of recurrent acute pancreatitis, chronic pancreatitis, and chronic pancreatic-type pain associated with PD (Table 51.4). In the largest published series by Warshaw and colleagues (1990), 88 patients with recurrent acute pancreatitis (49%) or “pancreatic pain” (51%) had minor papilla sphincteroplasty with a mean follow-up of 53 months; 70% of patients were reported to show improvement, 85% if the minor papilla was stenotic at surgery, compared with 27% if it was not (P < .01). Of those with recurrent acute pancreatitis, 82% were reported to have improved compared with 56% in the chronic pain group (P < .01). Preoperative ultrasonography (US) with secretin stimulation was compared with examination of the minor papilla and had a sensitivity of 78% and a specificity of 97%. Thus preoperative US with secretin stimulation was judged to be a good predictor of surgical success (92% success if positive, 40% success if negative). Seven patients were documented to have developed a restenosis at the minor papilla, six of whom had further surgery. The study concluded that a demonstrable stenosis at the minor papilla was a necessary cofactor in the development of recurrent acute pancreatitis or pancreatic pain in patients with PD.
Surgical sphincteroplasty is not without risk, however. In a recent large series of 446 patients, complications were reported in 34.8% of patients; pancreatitis (8.8%), asymptomatic hyperamylasemia (6.0%), and wound/abdominal infection (7.1%) were the most common morbidities. One death occurred after a duodenal leak (Madura et al, 2005).
The Case Against an Association Between Pancreas Divisum and Pancreatitis
An argument is also made against the theory of minor papilla obstruction. It is argued that patients with pancreatitis secondary to PD should have a dilated ductal system, but this has not been shown to be the case in the majority of studies that have assessed for it (Fogel et al, 2007). Similarly, if pancreatitis were associated with PD, only the dorsal pancreas should be affected, yet ventral duct pancreatitis is present in up to 11.8% of patients with PD and is the only duct involved in 4.2% of patients.
Cystic Fibrosis and Recurrent Pancreatitis
Cohn and colleagues (1998) and Sharer and colleagues (1998) first described the link between cystic fibrosis gene mutations and idiopathic pancreatitis. More recently, Choudari and colleagues (2004) demonstrated that mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene were present in 8 (22%) of 37 patients with PD and pancreatitis compared with 0 of 20 patients with PD and no history of pancreatitis (odds ratio [OR], 11.8; confidence interval [CI], 8.9 to 14.7; P = .02). The authors concluded that in patients with PD, CFTR mutations increased the risk for pancreatitis. Furthermore, Gelrud and colleagues directly measured CFTR gene function in nasal epithelium in response to isoproterenol and demonstrated that those with PD and recurrent acute pancreatitis had results somewhere between those observed for healthy controls and those for classic cystic fibrosis patients. Thus CFTR dysfunction may explain why some PD patients have recurrent acute pancreatitis but others with PD do not. Moreover, of 12 patients with PD and recurrent acute pancreatitis, 10 had undergone therapy to the minor papilla, and only 2 had resolution of their symptoms.
Annular Pancreas
Annular pancreas is a rare congenital abnormality in which the head of the pancreas completely encircles the second part of the duodenum (Fig. 51.4). It was first described in 1862 by Ecker, who reported a “ring derived from the head of the pancreas which surrounded the descending portion of the duodenum and was formed by uninterrupted glandular tissue” (Ravitch & Woods, 1950). The prevalence in autopsy studies is approximately 3 in 20,000 (Ravitch & Woods, 1950) but is higher in patients undergoing ERCP, at approximately 3 in 1000 (Fogel et al, 2006). Given the difficulty in making the diagnosis during autopsy and the highly selected patient population undergoing ERCP, the true prevalence likely lies somewhere in between.
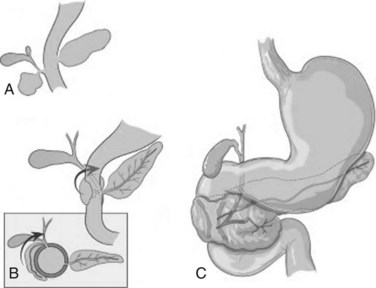
FIGURE 51.4 Development of annular pancreas.
(Modified from Zyromski, et al, 2008: Annular pancreas: dramatic differences between children and adults. J Am Coll Surg 206:1019-1025.)
Pathogenesis
In early development, the ventral pancreas is formed by two buds (see Chapter 1A). In mammals, the left ventral pancreatic bud is thought to regress (Lammert et al, 2001; Odgers, 1930), and the larger right ventral pancreatic bud migrates to take up the position seen in the adult. A number of hypotheses have been proposed to explain the occurrence of annular pancreas. The most persistent is that of Lecco (1910), who suggested that adherence of the right ventral pancreatic bud to the duodenum before gut rotation resulted in a partial or complete ring of pancreatic tissue around the duodenum. A competing hypothesis offered by Baldwin (1910) postulates that the left ventral bud persists to form the annulus. Although in most annular pancreas specimens, only one ventral lobe is apparent, reports of a bilobar ventral pancreas have surfaced that support Baldwin’s theory (Nobukawa et al, 2000). Neither of these theories explains the variation in the position of the annular duct seen in various specimens, and Kamisawa and colleagues (2001) proposed a new theory that the tip of the left ventral bud adheres to the duodenum and stretches to form a ring. The exact location of this attachment in relation to the bile duct determines the final arrangement of the annular duct.
Reports of familial annular pancreas support a genetic basis for the disease. Annular pancreas has been described in siblings (Claviez et al, 1995; Lainakis et al, 2005; Montgomery et al, 1971
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree