Components Separation
Derek E. Bell
Introduction
The massive ventral hernia presents a significant and poorly mastered reconstructive challenge (Fig. 29.1). Hernia develops in up to 11% of all primary laparotomies. It is estimated that over 250,000 ventral herniorrhaphies are performed each year in the United States. Recurrence rates of incisional herniorrhaphy are as high as 44% to 58%. Prosthetic mesh may lower recurrence rates but this is not without the increased rate of complications such as infection, extrusion, increased adhesions thus causing bowel obstruction or fistula formation. Primary closure has been found to have an unacceptable recurrence rate of up to 58%.
Ramirez first published an innovative technique for true reconstruction of the abdominal wall in 1990. This method entitled “component separation” utilized the rectus abdominis for reconstruction of midline defects. In this technique, the rectus abdominis is freed from the external oblique and in doing so this release allows for medialization of the rectus abdominis to the midline. The preliminary study which was performed on fresh cadavers allowed for medialization up to 10 cm of each muscle along the midline. This has become the basis for dozens of modifications of this technique since its first publication in 1990. This chapter will discuss the technique described by Ramirez, modifications of the technique and the benefits, patient selection, complications, and expected outcomes.
Even with component separation techniques, prior mesh repair has been demonstrated to be an independent risk factor for development of hernia recurrence. Repair of the massive ventral hernia can be performed with bioprosthetic or synthetic meshes although true repair of the coelomic defect is arguable. The reason for this is that repair with a multitude of meshes does not truly restore the dynamic function of the abdominal wall and predisposes the patients to complications such as fistulae, evisceration, infection, and death.
Anatomy
The rectus abdominis is a bi-pedicled muscle flap or Mathis and Nahai type III flap with a dual vascular supply originating from the deep superior and inferior epigastric arteries. The fibers are vertically oriented and are contained anteriorly by the anterior rectus sheath and deep or posteriorly by the posterior rectus sheath. The muscle is attached to
the costal margin cranially and to the pubis at the caudal extent. The arcuate line of the abdomen, Linea semicircularis or Douglas’ line is a horizontal line that demarcates the lower limit of the posterior layer of the rectus sheath. Inferior to this line, the internal oblique and transversalis pass anterior to the rectus sheath and generally this is also where the inferior epigastric vessels perforate the rectus abdominis. Superior to the arcuate line, the internal oblique aponeurosis split to envelope the rectus abdominis both superficial and deep to the muscle itself and helps to form the anterior and the posterior rectus sheath. The deep inferior epigastric originates from the internal iliac arteries and the superior epigastric arteries originate from the internal thoracic arteries otherwise known as the internal mammary arteries. The innervation to the rectus abdominis is via the intercostal nerves that traverse the abdominal wall in the plane between the internal oblique and transversalis.
the costal margin cranially and to the pubis at the caudal extent. The arcuate line of the abdomen, Linea semicircularis or Douglas’ line is a horizontal line that demarcates the lower limit of the posterior layer of the rectus sheath. Inferior to this line, the internal oblique and transversalis pass anterior to the rectus sheath and generally this is also where the inferior epigastric vessels perforate the rectus abdominis. Superior to the arcuate line, the internal oblique aponeurosis split to envelope the rectus abdominis both superficial and deep to the muscle itself and helps to form the anterior and the posterior rectus sheath. The deep inferior epigastric originates from the internal iliac arteries and the superior epigastric arteries originate from the internal thoracic arteries otherwise known as the internal mammary arteries. The innervation to the rectus abdominis is via the intercostal nerves that traverse the abdominal wall in the plane between the internal oblique and transversalis.
 Figure 29.1 Patient with a massive ventral hernia after exploratory laparotomy and split thickness skin grafting of the granulated visceral contents. |
The paired deep inferior epigastric arteries are the main blood supply for the abdominal wall. These are most commonly associated with two veins. These arteries often branch and have a variable aborization but generally remain deep to the rectus abdominis muscle caudal to the arcuate line. The branching system most commonly involves two major branches but may remain as a single dominant pedicle and least often as three branches. Eighty percent of the dominant blood supply is derived from the lateral branches of the deep inferior epigastric artery after its division. Perforating branches traverse the rectus abdominis to supply the skin surface. The perforators contribute to the subdermal plexus providing vascularity to the dermis and epidermis. The Cadaveric studies have revealed that very few perforators penetrate the linea alba or the external oblique aponeurosis and the greatest density of perforators is in the periumbilical region. Perforator sparing techniques have been employed as to minimize the risk of skin edge necrosis.
Timing of reconstruction has been of controversy. First and foremost there should be management of exposed visceral contents in the case of open abdomen. Patients who have undergone trauma laparotomies are treated with Vicryl or other absorbable mesh
or a biologic neodermis which helps to contain the visceral contents and create a smooth surface for granulation to occur. After a healthy granulation bed has formed, split thickness skin grafting of the granulating wound can be performed. A wound VAC device helps to optimize the take of the graft and provide uniform compression of the graft to the wound bed during the postoperative graft incorporation process and this should not preclude weaning or extubating a patient if the patient’s pulmonary dynamics would allow doing so. The overall minimization of perioperative complications with control of the open abdomen in this manner has been advocated. Planned, staged component separation reveals major complication rates to be acceptably low with recurrence of 5%. With this conservative approach to reconstruction, mortality is extremely low and approaching 0% at 24-month follow-up. Grafting of the coelomic contents, however, provides little strength or structural support otherwise to the abdominal wall. Loss of support of the abdominal wall centrally, with or without necessity of grafting, over time allows for the fascial defect to increase as the vector of the abdominal wall musculature is in a lateral and a posterior direction. In contrast to staged management of the open abdomen, patients undergoing early fascial closure in trauma patients have dismal results with mortality approaching 30%.
or a biologic neodermis which helps to contain the visceral contents and create a smooth surface for granulation to occur. After a healthy granulation bed has formed, split thickness skin grafting of the granulating wound can be performed. A wound VAC device helps to optimize the take of the graft and provide uniform compression of the graft to the wound bed during the postoperative graft incorporation process and this should not preclude weaning or extubating a patient if the patient’s pulmonary dynamics would allow doing so. The overall minimization of perioperative complications with control of the open abdomen in this manner has been advocated. Planned, staged component separation reveals major complication rates to be acceptably low with recurrence of 5%. With this conservative approach to reconstruction, mortality is extremely low and approaching 0% at 24-month follow-up. Grafting of the coelomic contents, however, provides little strength or structural support otherwise to the abdominal wall. Loss of support of the abdominal wall centrally, with or without necessity of grafting, over time allows for the fascial defect to increase as the vector of the abdominal wall musculature is in a lateral and a posterior direction. In contrast to staged management of the open abdomen, patients undergoing early fascial closure in trauma patients have dismal results with mortality approaching 30%.
Patients with midline ventral hernias generally have a spectrum in the quality of the skin overlying the fascial defect. Adhesions must be given sufficient time to soften in order to easily obtain adhesiolysis and minimize the risk of inadvertent enterotomy. Patients who have undergone grafting of the viscera, this can be easily apparent by pinching the skin and elevating this away from the visceral content to determine the pliability from the underlying tissues. This would suggest that adhesiolysis could most easily be undertaken and in doing so minimize the risks of complications such as inadvertent enterotomy and fistulae (Fig. 29.2). In patients who have native attenuated skin along the midline, safe, cutaneous closure after resection can be determined by simply pinching the skin together along the midline. It is imperative that quality and pliability of the anticipated remaining skin needs to be considered as to obtain a tension-free cutaneous closure. Failure to do so will usually result in wound healing problems, dehiscence, and potential major complications. In obese patients, weight loss should be advocated to enhance the pliability of the overlying skin with loss of the subcutaneous adipose tissue and also optimize a successful reconstruction by eliminating visceral obesity as a risk for hernia recurrence.
It is a personal preference to obtain a preoperative CT scan of the abdomen and pelvis with both oral and intravenous contrast. This allows for preoperative planning as the rectus can be evaluated for both viability and distance from the midline. Distances of over 20 cm will likely require an interposition mesh or can be obtained with overlapping of the fascia of the anterior rectus sheath. Often patients have undergone a multitude of prior surgeries which will compromise the vascularity of the rectus abdominis or the overlying skin. Evaluating the superficial and deep epigastric arteries with CT
pre-operatively is important as to avoid compromise of the abdominal wall skin postoperatively. In a circumstance where one of the epigastric vessels was compromised, a formal bilateral component separation should be avoided as to minimize failure of the repair, early wound dehiscence, and necrosis of the abdominal wall. Additionally, planning of the type or one of the several modifications of the component separation technique can be anticipated preoperatively. A CT defines the visceral anatomy within the hernia and may direct the safest approach for entering the coelomic cavity.
pre-operatively is important as to avoid compromise of the abdominal wall skin postoperatively. In a circumstance where one of the epigastric vessels was compromised, a formal bilateral component separation should be avoided as to minimize failure of the repair, early wound dehiscence, and necrosis of the abdominal wall. Additionally, planning of the type or one of the several modifications of the component separation technique can be anticipated preoperatively. A CT defines the visceral anatomy within the hernia and may direct the safest approach for entering the coelomic cavity.
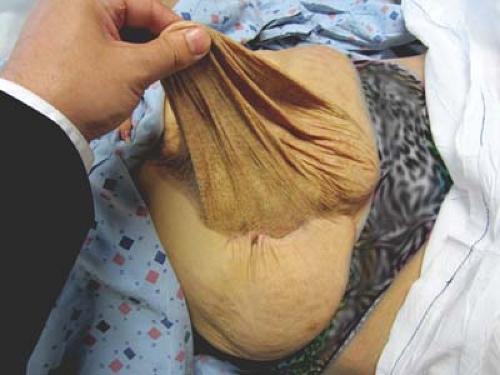 Figure 29.2 Pinch test demonstrating the pliability of the skin separation from the underlying adhesions. |
Debridement of necrotic or infected tissues including infected or exposed meshes should be undertaken prior to definitive repair. Wound infection significantly increases the risk of hernia recurrence to as much as 80%. Some authors advocate for control of ostomies or fistulae by restoring enteric continuity if planned in a staged fashion as well. The challenge this creates is that individualized patient anatomy may not allow for skin closure after such a procedure, so this must be taken into consideration.
Smoking cessation should be emphasized prior to surgery as to minimize skin edge necrosis and wound healing problems. In obese patients, weight loss should be considered and optimization of nutrition should be achieved preoperatively as well.
There are no formal indications for abdominal wall reconstruction via component separation technique; however, there are a multitude of instances when this treatment modality for abdominal wall reconstruction should be considered. General consideration for employing this technique includes large midline hernias, infected wounds or those that have exposed mesh and patients who have failed prior herniorrhaphy. The number of failed attempts at herniorrhaphy directly correlates with likeliness of additional failures with conventional mesh techniques and approaches 50% after three repairs.
One of the benefits of this technique is that autologous tissues are used. Thus, in wounds such as those with attempts with synthetic mesh repair and exposure or active infection of the mesh is noted, component separation is a good option for reconstruction. Most commonly this does not require the use of any synthetic or biologic mesh at all. Therefore, abdominal wall reconstruction can be undertaken in non-clean fields such as those with enterocutaneous fistulae or ostomy reversals. Some sources advocate for closure of fistulae, infection or reversal of ostomies in a preliminary procedure, and thus a staged fashion to prevent complications before undertaking definitive reconstruction. This proposed staged reconstruction would require intentionally leaving the patient with a hernia by either closing the skin only or placing a skin graft over granulated bowel or over a vascularized bioprosthetic mesh. The use of synthetic mesh is a relative contraindication with patients classified as having contaminated or dirty wounds and should be avoided in herniorrhaphy requiring such and a biologic mesh should be considered.
The component separation technique medializes the rectus abdominis and in doing so provides highly vascularized, neuritized dynamic muscular support to the midline. It has been postulated that by restoring the dynamic support across the hernias, the intrinsic weakness of this area is distributed along the entirety of the abdominal wall. Muscular closure eliminates this focal point of weakness although mesh repair does not. With this philosophy, many experts advocate that any ventral hernia should be repaired with muscle.
Defect size is of debate as to the appropriate approach to repair and minimization of recurrence rate. Mathes advocates for defects greater than 40 cm2, while Shestak uses 6 cm as the arbitrary defect diameter for performing component separation. In a prospective analysis of sutured versus meshed repair of hernias greater or less than 10 cm2, a failure rate of those hernias greater than 10 cm2 was 63% in the sutured group versus 32% in the meshed repair. The group with defects less than 10 cm2 the recurrences were greater than 17% with either repair. All modalities based upon Burger’s analysis are of notable risk for recurrence based regardless of the size of the defect and arguably these patients may have lower recurrence with a component separation reconstruction of the abdominal wall.
The professional consensus is that any patient who has failed prior repair, and especially multiply failed repairs, should be considered for component separation. Poor viability of midline tissues such as the fascia warrants medialization of high quality tissues and releases to minimize tension at the midline. This should be taken into consideration in patients with lesser quality tissues such as those that are immunocompromised, diabetic, or older individuals in attenuated tissues require the tension-free repair that component separation provides.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


