Chapter 40 Complications of Assisted Reproductive Technologies
OVARIAN HYPERSTIMULATION SYNDROME
OHSS is the most common and potentially serious complication of ovarian hyperstimulation for ART procedures, especially in vitro fertilization (IVF). This syndrome consists of ovarian enlargement in conjunction with a continuum of symptoms, depending on severity (Fig. 40-1). In the mildest cases, the patient experiences only pelvic discomfort and nausea. More serious cases are associated with vomiting, abdominal distension, and ascites. The most severe cases can result in respiratory distress, oliguria, hemoconcentration, and thrombosis. In rare cases, death has been reported.
Incidence
The vast majority of OHSS cases occur in association with ovarian stimulation with injectable gonadotropins. Only occasional cases have been observed after clomiphene citrate administration, and very rare cases have been reported during the first trimester of pregnancy resulting from spontaneous unstimulated ovulation.1,2 Sporadically reported cases of familial spontaneous OHSS may be due to a mutation in the follicle-stimulating hormone (FSH) receptor, leading to hypersensitivity of the corpus luteum to human chorionic gonadotropin (hCG).3,4 However, the great majority of cases develop after use of injectable gonadotropins for ovarian hyperstimulation before in vitro fertilization (IVF).
The actual frequency of OHSS varies depending on patient factors, surveillance methods, and management approaches. Mild cases occur in as many as 30% or more of patients undergoing controlled ovarian hyperstimulation with gonadotropins for IVF and often go unrecognized due to the benign nature of the signs and symptoms.5 In general, less than 5% of patients undergoing IVF would be expected to develop moderate symptoms and less than 1% will develop severe symptoms.6,7 It remains unclear why many high-risk induction cycles do not result in severe OHSS while others do.
Pathogenesis
OHSS is the result of excessive follicular response during the follicular phase, but manifests exclusively during the luteal phase following a surge in luteinizing hormone (LH) or ovulatory dose of hCG. The severity and duration is intensified by additional doses of exogenous hCG for luteal support and rising levels of endogenous hCG if the patient becomes pregnant.1,5,8
Vasoactive Substances
Possible participants in the pathogenesis of OHSS include vascular endothelial growth factor (VEGF), prostaglandins, other members of the cytokine family, and nitric oxide, as well as components of the renin and the angiotensin system, especially angiotensin II.9–13 All of these are recognized participants in the normal physiology of folliculogenesis and luteogenesis.14,15
The primary mediator of increased vascular permeability associated with OHSS appears to be the cytokine VEGF.9,10 VEGF is secreted by granulosa and theca cells in the late follicular phase. Neoangiogenesis, essential to folliculogenesis and especially to luteogenesis, is induced largely by VEGF.11–13
Ovarian hyperstimulation syndrome is most commonly thought to be the result of vascular hyperpermeability caused by excessive VEGF-induced changes associated with extreme neoangiogenesis. The expression of VEGF by granulosa cells has been shown to be up-regulated by hCG, and unbound levels of VEGF correlate with the severity of OHSS.16,17 Inhibition of VEGF is associated with improvement in vascular permeability.18,19 Evidence indicates that supernumerary follicles are the predominant source of superphysiologic quantities of VEGF in patients who develop OHSS.20 VEGF acting directly or indirectly in concert with other factors may diffuse into the peritoneal cavity, resulting in increased permeability of diffuse mesothelial vessels. Removal of these substances by paracentesis may facilitate reduction of capillary hyperpermeability, resulting in the observed improvement in the clinical sequelae.21
Fluid Homeostasis
The hemoconcentration associated with OHSS leads to hypercoagulability and increased risk of thromboembolism, especially if it occurs in combination with thrombophilias or other coagulopathies.22,23 There is also evidence that OHSS may induce a primary hypercoagulable state independent of hemoconcentration.24
The hypovolemia resulting from OHSS can lead to low blood pressure and decreased central venous pressure, followed by decreased renal perfusion. Decreased renal perfusion results in increased sodium and water reabsorption in the proximal tubule and reduced exchange of hydrogen and potassium for sodium in the distal tubule, potentially leading to oliguria with prerenal azotemia, hyponatremia, and hyperkalemic acidosis.25 Medical complications secondary to OHSS include renal insufficiency, adult respiratory distress syndrome, hepatocellular damage, hypovolemic shock, disseminated intravascular coagulation, and thromboembolism.5
Risk Factors
No single clinical characteristic or combination of characteristics is 100% predictive of OHSS. However, the incidence is increased by any factor that increases ovarian response (Table 40-1). Patients at highest risk for severe OHSS are those who meet the folowing criteria:
Table 40-1 Risk Factors for Ovarian Hyperstimulation Syndrome (OHSS)
| Younger age |
| Low body mass index |
| Polycystic ovary syndrome |
| hCG luteal phase supplementation |
| Previous OHSS |
For patients with both of these risk factors, the incidence of severe OHSS has been reported to be as high as 80%.26
The occurrence of OHSS is more closely correlated with degree of ovarian stimulation than it is with the dose and duration of gonadotropins.27–29 OHSS occurs more commonly in women with ovaries that are highly sensitive to FSH stimulation, specifically young women and women with polycystic ovary syndrome (PCOS).28,30 Higher doses of gonadotropin are also associated with an increased incidence of OHSS, but only to the extent that higher doses result in more vigorous ovarian stimulation.29 The presence of a large number of follicles, particularly small and moderate-sized follicles, at the time of initial hCG is a positive risk factor for OHSS.2 Estrogen produced by the developing follicles serves as a marker of the degree of ovarian hyperstimulation. High estrogen levels in excess of 4000 pg/mL are of particular concern in the presence of numerous small to medium-sized follicles in contrast to a smaller number of exclusively large or mature follicles.1,28,31
Clinical Symptoms
Classification
OHSS is best thought of as a broad continuum of signs and symptoms.27,28 However, for management and reporting purposes, OHSS is generally classified as mild, moderate, or severe (Table 40-2). Mild OHSS is a self-limited and clinically benign condition characterized by ovarian enlargement (generally less than 5 cm), abdominal bloating, and discomfort. Moderate OHSS indicates progressively greater ovarian enlargement and significant symptoms that are difficult to manage outside a hospital environment. Severe OHSS involves massive cystic ovarian enlargement in excess of 10 cm and tense abdominal ascites with or without pleural effusion.27
Table 40-2 Classification of Ovarian Hyperstimulation Syndrome
Modified from Navot D, Bergh PA, Laufer N: Ovarian hyperstimulation syndrome in novel reproductive technologies: Prevention and treatment. Fertil Steril 58:249–261, 1992.
Onset of Symptoms
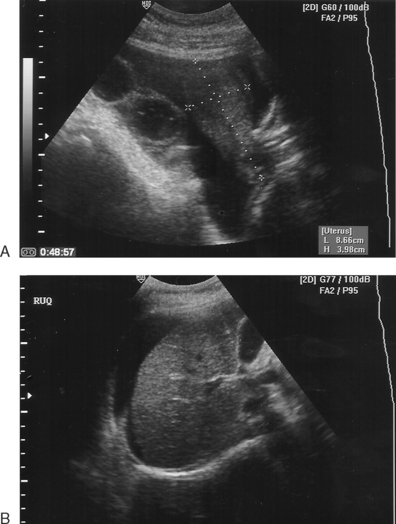
Most commonly, patients present with complaints of abdominal bloating, shortness of breath, nausea, unusual weight gain, and decreased urine output. Patients with severe OHSS appear ill, with marked abdominal distension secondary to ascites. They frequently become significantly short of breath in the supine position, again secondary to abdominal ascites. Laboratory findings include elevated hematocrit, leukocytosis, hyponatremia, hyperkalemia, elevated blood urea nitrogen (BUN)-to-creatinine ratio, and occasionally mildly elevated results on liver function tests. On ultrasound, ovaries will be large and cystic with variable amounts of free peritoneal fluid filling the abdominal space. Occasional patients will present with a pleural effusion, most commonly right sided, with or without abdominal ascites.29
Prevention
Avoidance of Excessive Stimulation
If rapidly rising estrogen levels reach or threaten to become unacceptably high, withholding the daily dose of gonadotropin can reduce the incidence and severity of OHSS. This approach, coasting, can in some cases result in arrest and atresia of all or most of the follicles; however, in many cases hCG administration can be delayed until the estradiol level returns to a more acceptable level without a detrimental effect on the subsequent oocyte and embryo quality or pregnancy rate.33–36 Coasting has been shown to be associated with reduced concentrations of follicular VEGF and a significantly lower incidence of severe OHSS.37 Coasting is most successful when the serum estradiol has exceeded 3000 pg/mL and the lead follicle has reached a diameter larger than 14 mm. If growth of the lead follicles continues, administration of hCG can be withheld for up to 4 days until the serum estradiol reaches an acceptable level. Prolonged coasting beyond 4 days is associated with a decrease in implantation and pregnancy rates.38
Withholding hCG
Because the clinical signs and symptoms of hyperstimulation will not develop until final follicular maturation and luteinization occur in response to hCG or LH, it is most prudent to withhold the ovulatory dose of hCG in patients who are clearly overstimulated based on follicular number, size, and estrogen level.31,33 Although the decision to drop a cycle ultimately becomes a matter of clinical judgment, in general it is our practice to withhold hCG in the presence of a large number of small follicles and a peak estradiol level in excess of 4000 pg/mL.
Intravenous Albumin
Intravenous administration of an osmotically active “colloid” agent at the time of retrieval appears to reduce the risk and severity of OHSS in high-risk patients. Administration of intravenous albumin (25 g) during and immediately after the egg retrieval has been recommended for women with a peak estradiol level of 3000 pg/mL or a large number of small and intermediate-sized follicles.39 Based on a meta-analysis using the Cochrane standard, it is estimated that for every 18 women treated with albumin, 1 case of OHSS will be avoided.40 However, prophylactic administration of albumin to high-risk patients remains controversial because of conflicting data regarding its efficacy as well as concerns regarding the expense and safety.41
The mechanism of action for intravenous albumin is not clear. It was initially hypothesized that albumin exerts it effect by increasing intravascular osmotic pressure. However, because of its small molecular weight, albumin is cleared from the circulation within a short time of the retrieval. Other possible mechanisms include increased plasma binding of a factor or factors involved in the pathogenesis of OHSS. Other osmotic agents have also been used with some success, including 5% hydroxyethylstarch and 3.5% degraded gelatin polypeptides (Haemaccel).42–44
Luteal Phase Support
Introduction of a GnRH antagonist early in the luteal phase will lead to early luteolysis.45 Because OHSS resolves with involution of the corpora lutea, the clinical course of severe OHSS can be abbreviated or even eliminated by this approach. If the stimulation protocol did not include down-regulation with a GnRH agonist, the gonadotropin flare secondary to initiation of a GnRH agonist can be used to induce oocyte maturation and ovulation followed almost immediately by irreversible luteolysis.18,46–51
Pituitary response to initiation of a GnRH agonist is preserved following a GnRH antagonist/gonadotropin stimulation protocol. If embryos are transferred in a cycle in which premature luteolysis is induced, exogenous hormone replacement is required to support endometrial maturation and implantation.52 Alternatively, the embryos can be cryopreserved for transfer at a later time.53
Management of OHSS
Diagnosis
Proactive management of patients with early signs and symptoms of OHSS can ameliorate the severity of OHSS.54 Patients generally present with complaints of abdominal bloating, shortness of breath, and nausea. Symptomatic patients should be seen and evaluated promptly. Clinical parameters should include a physical examination, including a chest and abdominal examination (but not a bimanual pelvic examination) as well as a pelvic or abdominal ultrasound for ovarian enlargement or ascitic fluid and to rule out torsion.
Laboratory studies should include complete blood count, electrolytes, BUN, and creatinine. Hemoconcentration (hematocrit >45%) heralds the development of ascites, marginal renal perfusion, and decreasing urine output and increases the risk of thromboembolic events.55
Most patients with OHSS will have some degree of ascites (Table 40-3). Less commonly, patients will develop pleural effusion, which can occur unilaterally (usually on the right) and in the absence of significant abdominal ascites. A high index of suspicion and careful evaluation are important to differentiate this condition from pulmonary embolism.32
Table 40-3 Complications Associated with OHSS
| Ascites |
Get Clinical Tree app for offline access 
|