INTRODUCTION
Screening for colorectal cancer (CRC) can reduce disease-related morbidity and mortality. The existing evidence has led to the recommendation of CRC screening as a standard component of preventive health care.
CRC is the third leading cause of cancer death in the United States and Western Europe. The lifetime risk of CRC in the United States is approximately 5%. In 2015, it is estimated that 69,090 men and 63,610 women would be diagnosed and 49,700 individuals would die of CRC. However, overall disease survival has improved from 51.4% in the 1970s to 65% in 2010. Increased knowledge about the pathogenesis, advances in medical and surgical care, and the increasing emphasis on CRC screening programs have contributed to these substantial gains.
CRC can be prevented through readily available screening. Prevention efforts rely on the long time interval required for a benign adenomatous polyp to progress into an invasive cancer. It is estimated that the adenoma to carcinoma sequence unfolds over a 7- to 10-year period. In addition, CRC-related deaths are preventable if the disease is detected early. When diagnosed early, the 5-year survival rate for CRC that is still confined to the primary site (localized stage) approaches 90%. Conversely, the corresponding 5-year survival for patients with known distant metastases is only 13%. Unfortunately, only 40% of cancers are diagnosed at an early stage and up to 20% of patients have distant metastases.
Nevertheless, CRC screening is presently underutilized in the United States. Less than two-thirds of the population is currently compliant with standard screening recommendations, despite the array of available choices. CRC screening lags far behind screening for other common malignancies, such as breast, cervical, and prostate cancer. According to recent results by the National Center for Health Statistics, only 65% of Americans aged 50 years or older had undergone any type of CRC screening and only 42% had undergone screening within the recommended time interval. At present, issues of sensitivity, specificity, and patient acceptance limit existing CRC screening methods. Several factors contribute to the lack of compliance with CRC screening, including inappropriate perception of risk (particularly if patients are asymptomatic and without a family history of CRC), dietary restrictions or burdensome cathartic preparations, the invasiveness of procedures, and perceived discomfort, pain, and embarrassment related to certain screening techniques.
[PubMed: 19998273]
[PubMed: 15139050]
[PubMed: 24399786]
Three distinct molecular pathways by which genetic and epigenetic events may lead to CRC have been recognized: the chromosomal instability (CIN), the microsatellite instability (MSI), and the CpG island methylator phenotype (CIMP) pathways.
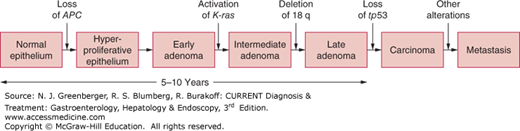
In 1990, a stepwise, chronologic model for colorectal tumorigenesis was proposed. It outlined sequential alterations in key growth regulatory genes, such as APC, K-ras, and tp53, culminating in the development of a malignant neoplasm. This pathway contributes to the development of 60% of sporadic colorectal tumors that arise from preexisting adenomatous polyps. When adenomas accumulate the necessary combination of genetic mutations in the suggested stepwise manner, the end result is cancer (Figure 22–1).
The genes implicated in CRC can be divided into three categories: (1) tumor-suppressor genes, (2) proto-oncogenes, and (3) DNA repair genes.
The function of tumor-suppressor genes is to downregulate normal growth stimulatory pathways. In CRC, the genes most frequently inactivated are APC, tp53, and p16. Consistent with the Knudson “two-hit hypothesis,” acquired (or somatic) mutations in both alleles of the tumor-suppressor gene are required to fully inactivate gene function and cause cancer. In autosomal-dominant CRC syndromes, a preexisting germline mutation in one allele (“first hit”) is inherited and a “second hit” occurs when an acquired mutation inactivates the other allele.
Proto-oncogenes, such as K-ras, are components of signaling pathways that promote normal cellular growth and proliferation. A mutation in a proto-oncogene leads to an active gene product with a resulting tumorigenic effect.
DNA repair genes function to maintain the integrity of the genome. Individual nucleotides can be modified by biochemical reactions such as oxidation, alkylation, spontaneous deamination, and ultraviolet cross-linking. When errors occur, they are corrected through a sophisticated process known as “base excision repair.”
Another manner in which errors are introduced into the genome is through mispairing of nucleotides, which can occur during normal DNA replication. These errors are corrected by a DNA “mismatch repair” (MMR) system. When either of these DNA repair processes is dysfunctional, deleterious mutations can accumulate in genes that directly control cellular growth and proliferation. Germline mutation in MMR genes is responsible for Lynch syndrome, while somatic mutation or hypermethylation silencing of MMR genes accounts for approximately 15% of sporadic CRC.
Epigenetic alterations which alter gene expression or function without altering the DNA sequence can occur. Aberrant hypermethylation of gene promoter regions (CpG islands) may result in gene silencing of tumor suppressor genes. CIMP-high CRCs are strongly associated with BRAF mutations, developing from a precursor lesion known as the sessile serrated adenoma (SSA). CRCs arising along the serrated pathway account for about 10% of all CRC.
[PubMed: 22694276]
[PubMed: 2188735]
[PubMed: 20420948]
[PubMed: 20420946]
RISK STRATIFICATION
The approach to CRC screening in asymptomatic individuals depends on risk stratification and the likelihood of developing the disease. Screening for CRC takes place in persons who have no personal or family history of adenomatous polyps or cancer. Surveillance takes place in persons with a personal history of either adenomatous polyps or CRC, or one of the genetic syndromes that requires more intensive monitoring. Individuals who have signs or symptoms suggestive of CRC fall outside the domain of screening and should be offered appropriate diagnostic evaluation.
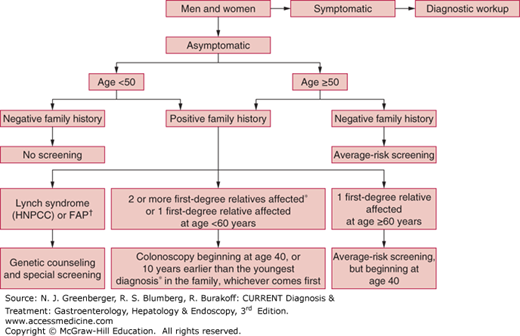
CRC screening programs begin by classifying an individual’s level of risk based on age, as well as personal and family medical history (Figure 22–2). This vital information helps determine when screening should be initiated, with what appropriate tests, and the frequency of subsequent examinations. CRC risk is commonly stratified into three broad categories: average risk, moderate risk, and high risk.
Figure 22–2.
Algorithm for colorectal cancer screening. FAP, familial adenomatous polyposis; HNPCC, hereditary nonpolyposis colorectal cancer; *, either colorectal cancer or adenomatous polyps; †, see text. (Adapted, with permission, from Winawer SJ, Fletcher R, Rex D, et al. Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rational–update based on new evidence. Gastroenterology. 2003 Feb;124(2):544-560.)
SCREENING & SURVEILLANCE RECOMMENDATIONS
An average-risk individual is defined as an asymptomatic person without a personal or family history of colonic polyps or cancer. The majority of the general population is considered to be at average risk for CRC. Given that age is the strongest risk factor for the development of CRC and adenomatous polyps, men and women at average risk should be offered screening beginning at age 50 years.
Available screening options for average-risk individuals fall into two broad categories: (1) tests that primarily detect CRC, and (2) those that detect precancerous adenomatous polyps and CRC (Table 22–1). Tests that primarily detect cancer are fecal tests, including guaiac-based and immunochemical occult blood tests, and fecal DNA tests. These modalities provide limited opportunity for prevention because detection of premalignant adenomatous polyps is most often incidental. Tests that detect both adenomatous polyps and cancer include flexible sigmoidoscopy (FS), colonoscopy, and imaging modalities including double-contrast barium enema (DCBE) and computed tomography colonography (CTC). Because prevention is the primary goal of CRC screening, recent guidelines from the American Cancer Society, the US Multisociety Task Force on Colorectal Cancer, and the American College of Radiology strongly encourage clinicians to offer those tests that are designed to detect both early cancer and adenomatous polyps if resources are available and if patients are willing to undergo an invasive examination. Noninvasive tests must be repeated at regular intervals to be effective, are less likely to prevent CRC than invasive examinations, and, if abnormal, require colonoscopy.
Tests that detect adenomatous polyps and CRC Flexible sigmoidoscopy Colonoscopy Double-contrast barium enema Computed tomography colonography |
Tests that primarily detect CRC Guaiac-based fecal occult blood testing Fecal immunochemical tests Stool DNA |
Nevertheless, health care providers are encouraged to focus on increasing screening rates through periodic use of any of the recommended modalities. Providers should present their patients with information about the advantages and disadvantages associated with the multiple available screening tests. In turn, patients have the opportunity to select how they wish to be screened based on their own preferences. The rationale for such an approach is that it may increase the likelihood that screening will occur. Even though the currently available screening techniques are not equal in effectiveness, cost, or associated risks, they all have been demonstrated to be cost-effective compared with no screening at all.
[PubMed: 18384785]
[PubMed: 10735014]
[PubMed: 12557258]
Individuals with a personal or family history of adenomatous polyps or CRC are considered to be at intermediate risk for the development of CRC. For these patients, colonoscopy is the recommended screening method. Other conventional screening modalities are not typically recommended in this setting.
Certain characteristics of colorectal adenomas at baseline colonoscopy are the basis for decisions about surveillance intervals. Existing data from the National Polyp Study (NPS), a pooled analysis of chemoprevention studies, as well as observational cohort studies support the presence of the following predictors for the development of future advanced adenomas or cancers as high-risk adenomas (HRAs): (1) three or more adenomas, (2) adenoma size greater than 1 cm, and (3) adenoma with villous features or high-grade dysplasia. Indeed, a recent Norwegian registry study demonstrated increased colorectal cancer mortality in individuals with high-risk adenomas (adenomas with high-grade dysplasia, a villous component, or size >10 mm). A proximal location of adenomas (ascending colon or cecum) may also predict metachronous advanced adenomas, but this has not been well studied to date. In addition, there is a general consensus among many of the studies that individuals with adenomas with lesser findings (eg, one or two subcentimeter adenomas without high-grade dysplasia or villous features) are at lower risk for subsequent advanced adenomas (or low-risk adenomas, LRAs).
By stratifying patients into “lower risk” and “higher risk” groups for future advanced adenoma development based on findings from initial colonoscopy, the NPS has provided evidence-based guidelines for surveillance of postpolypectomy patients. In a randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps, there was no better detection of advanced lesions with surveillance examination 1 year after the initial colonoscopy than with follow-up examination in 3 years. The inference made is that the rate of developing metachronous adenomas with advanced pathology is slow; therefore, the current recommendation for patients with a personal history of three or more adenomas, adenomas of 1 cm or larger, or adenomas with villous or high-grade dysplasia, or any combination of these findings, is surveillance colonoscopy in 3 years. In addition, patients who have more than 10 adenomas removed during one endoscopic examination should be examined in a shorter time interval (<3 years) based on clinical judgment and should be considered for the possibility of an underlying familial syndrome. The standard of care for a large sessile or malignant polyp removed by piecemeal fashion is 3–6 months after the initial endoscopic resection.
However, it is important to acknowledge that the timing of surveillance colonoscopy in patients with a history of adenomatous polyps is still evolving. The existing studies assessing subsequent risk for neoplasia after colonoscopic polypectomy have followed patients for 8–10 years or longer. Prior projections that patients without advanced adenomas can wait at least 5 years (and perhaps up to 10 years) for repeat colonoscopy have been validated in numerous studies, due to the small, insignificant increased risk of advanced neoplasia within 5 years when compared with individuals with no baseline neoplasia. Further studies are needed to stratify risk in this “low risk” group with diminutive (<6 mm) versus small (6–9 mm) polyps in order to confirm the strategy of these intervals.
The effectiveness of CRC prevention with colonoscopy assumes that the baseline examination is performed with high quality, as lesions may be missed and interval cancers can develop, notably in the proximal colon. Numerous factors may impact the detection of neoplasia in the proximal colon including the quality of the bowel preparation, subtle nature of flat lesions, and tumors which may be biologically different from the more prevalent neoplasia in the distal colon.
[PubMed: 22763141]
[PubMed: 25162886]
[PubMed: 8247072]
Individuals with a history of CRC are at risk for recurrent cancer and metachronous neoplasms and require endoscopic surveillance after surgical resection. Anywhere from 2% to 7% of patients with CRC have one or more synchronous cancers in the colon or rectum at the time of initial diagnosis; it is therefore important to perform a complete colonoscopy in the preoperative period. In cases where an obstructing colonic or rectal lesion is detected, CTC or DCBE should be considered perioperatively, and a complete colonoscopy should be performed 3–6 months after surgery. Once the colon is cleared of any synchronous lesions, a postoperative surveillance colonoscopy is recommended at 1 year to evaluate for any metachronous lesions. If the examination at 1 year is normal, the subsequent examination should be at 3 years. If this examination is also found to be normal, surveillance colonoscopy can thereafter be extended to every 5 years.
Colonoscopy at 1 year after surgical resection is recommended based on reports of a high incidence of metachronous second cancers noted within the first 2 years after resection. Among an aggregate of studies reporting results from postcancer surveillance colonoscopy, there was an incidence rate of 0.7% metachronous cancers in the first 2 years after resection of the initial primary cancer. This estimate is consistent with data from a tumor registry review in Nebraska, which calculated an annual incidence of 0.35% per year for metachronous cancers. This is considered sufficient information to warrant a colonoscopy at 1 year following surgical resection. However, this should not diminish the importance of a high-quality colonoscopic examination in the perioperative period to exclude synchronous neoplasms.
In addition, it is important to distinguish between rectal and colon cancer because of the differing rates of local recurrence. The recurrence of colon cancer at the anastomotic site occurs in only 2–4% of patients. In contrast, local recurrence rates of rectal cancer when patients have undergone a low anterior resection can be 10 or more times higher. High recurrence rates of rectal cancer are partly a function of surgical technique. Local recurrence rates of cancer can be reduced by using a surgical technique called mesorectal excision, as well as administering radiation and chemotherapy in the neoadjuvant, preoperative setting to patients with locally advanced disease. However, because reported local recurrence rates for rectal cancer across the United States are generally higher than those achieved in case series using total mesorectal excision, there is a rationale for performing periodic examinations of the rectum. Although effectiveness has not been proven, performing proctoscopy at 6-month intervals for the first 2 years after surgical resection can be considered for the detection of a surgically curable recurrence of the original rectal cancer in those patients who do not receive pelvic irradiation.
When colon or rectal cancer is endoscopically resected and surgery is not needed, follow-up endoscopic examination to inspect the biopsy site within 1 year is a reasonable strategy. As previously noted, colonoscopy is considered the test of choice for the detection of metachronous neoplasms in patients with a history of CRC. CTC has not been evaluated adequately in this setting, and guaiac-based fecal occult blood testing (FOBT) has been considered to have a very low positive predictive value after colonoscopic evaluation.
[PubMed: 24220554]
[PubMed: 16737948]
[PubMed: 16697750]
An individual’s risk of CRC is increased if there is a family history of adenomatous polyps or CRC. The screening recommendations based on familial risk are derived largely from the observed colon cancer risk in relatives of patients with CRC and adenomas diagnosed before age 60 (Table 22–2). Data from a meta-analysis of 27 studies assessing familial risk of CRC and adenomatous polyps report a relative risk of CRC when a first-degree relative was affected with CRC to be 2.4. The relative risk for CRC if the first-degree relative had an adenomatous polyp was 1.9, with age effects similar to those observed for cancer. In addition, if more than one relative was affected and CRC was diagnosed before age 45, the relative risk increased to 4.2 and diminished to 2.2 and 1.8 for ages 45–59 and older than 59 years, respectively. A recent study demonstrated that a greater risk of CRC was estimated for first-degree relatives if CRC cases had proximal colon cancers or if their tumors demonstrated pathologic features such as peritumoral lymphocytes, tumor-infiltrating lymphocytes, expanding tumor margin, or a synchronous CRC. Therefore, familial risk needs to be readily identified and should prompt the early initiation of screening with colonoscopy.
At present, the US Multisociety Task Force on Colorectal Cancer recommends that for patients who report one first-degree relative under 60 years or two first-degree relatives of any age with adenomatous polyps or CRC, screening colonoscopy should start at age 40 or 10 years younger than the earliest diagnosis of an affected relative (whichever one comes first) and continue every 5 years (see Figure 22–2; Table 22–3). Screening should start at the same time for those patients with a single first-degree relative with CRC or adenomas diagnosed after age 60, but surveillance should be performed as for average-risk individuals. The rationale for starting screening at age 40 is that the incidence of CRC in these patients resembles the risk in persons with no family history but precedes it by approximately 10 years. In addition, a patient reporting a second- or third-degree relative with adenomatous polyps or CRC does not confer additional risk, and therefore, average-risk screening recommendations are sufficient.
| Assessed Risk | Age to Initiate | Test | Interval |
|---|---|---|---|
| Averagea |