CHAPTER 98 Colonic Motor and Sensory Function and Dysfunction
ANATOMY AND BASIC CONTROL MECHANISMS OF THE COLON AND ANORECTUM
STRUCTURE AND ACTIVITY OF COLONIC SMOOTH MUSCLE
ION CHANNELS IN COLONIC SMOOTH MUSCLE
The membrane of colonic smooth muscle cells contains a variety of ion channels, including several types of potassium channels, calcium channels, chloride channels, and nonselective cation channels.2 Although the exact physiologic roles of many of these ion channels are unknown, the high-threshold, voltage-operated calcium channels (l-type calcium channels) do play a crucial role in colonic muscle contractility. These channels open when the membrane potential of smooth muscle cells is depolarized beyond a voltage threshold, and they are responsible for the rapid upstroke of smooth muscle action potentials. The influx of calcium through l-type calcium channels during action potentials is a major trigger for activation of the contractile apparatus. It is not surprising that pharmacologic blockade of l-type calcium channels by dihydropyridine drugs such as nifedipine can reduce the contractility of colonic smooth muscle substantially. Release of calcium from intracellular stores, which is triggered by excitatory neurotransmitters, also may play a role in muscle contraction.
INTERSTITIAL CELLS OF CAJAL
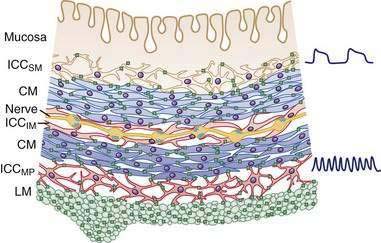
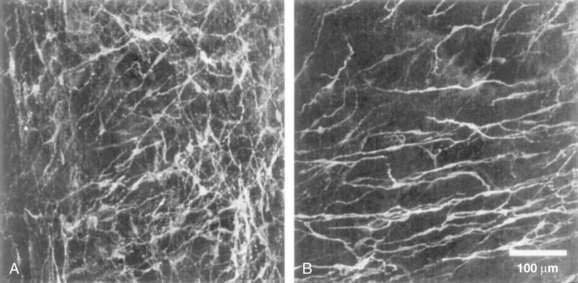
In the human colon, three types of ICC are recognized and are named according to their locations: ICC in the plane of the myenteric plexus (ICCMY), ICC near the submucosal plexus (ICCSM), and intramuscular ICC located between the circular and the longitudinal muscle layers (ICCIM). ICCMY and ICCSM form extensive networks along the colon and are electrically coupled to one another and to the smooth muscle layers by gap junctions (Figs. 98-1 and 98-2). ICCMY probably are the pacemakers for the small, rapid (12-20/min) oscillations in membrane potential (MPOs) of longitudinal and circular smooth muscle layers. ICCSM are the pacemakers for the large-amplitude slow waves (2-4/min) originating in the plane of the submucosal plexus; these slow waves have a powerful influence on the patterning of circular muscle contraction.
The discovery that cellular mechanisms long considered to be the properties of smooth muscle cells actually are mediated by ICC may have important clinical implications. For example, in the distal bowel, reduced numbers of ICC, or a reduction in the total volume of ICC, has been associated with anorectal malformations, colonic manifestations of Chagas’ disease, and possibly some cases of slow-transit constipation.3 Some reports have suggested that the density of ICC may be reduced in aganglionic segments of colon in Hirschsprung’s disease and that this deficit might contribute to diminished propulsive activity; this finding, however, has not been consistent between studies.3
INNERVATION OF THE COLON1
THE ENTERIC NERVOUS SYSTEM
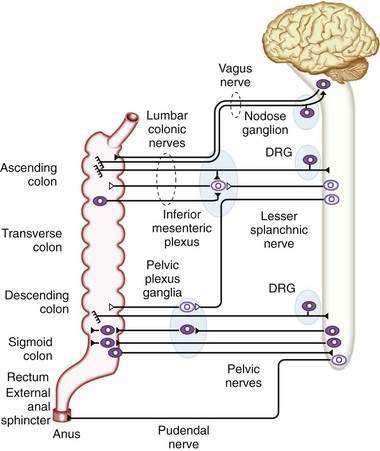
Direct neuronal control of colonic motility is mediated mostly by the enteric nervous system (ENS). Although the ENS is capable of expressing a diverse repertoire of motor patterns, its functions are modulated by sympathetic, parasympathetic, and extrinsic afferent pathways (Fig. 98-3). In terms of numbers of nerve cells, the ENS is by far the largest component of the autonomic nervous system, with considerably more neurons than those of the parasympathetic and sympathetic divisions combined. The nerve cell bodies of the ENS are located in plexuses of myenteric ganglia (Auerbach’s plexus), which lie between the longitudinal and the circular muscle layers of the muscularis externa, or in the submucosal ganglia, which lie between the circular muscle and the mucosa (Fig. 98-4).
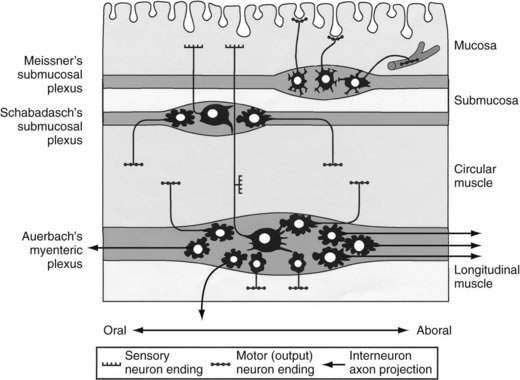
Figure 98-4. Diagram showing the layers and components of the intestinal wall. The lumen is at the top and the longitudinal muscle layer is at the bottom. Auerbach’s myenteric plexus and the submucosal plexuses (Meissner’s and Schabadasch’s plexuses) are shown, along with some of their major classes of enteric neurons. The networks of interstitial cells are shown in Figure 98-1.
Motor Neurons
Enteric motor neurons typically have smaller cell bodies than afferent neurons, with a few short dendrites and a single long axon. Separate populations of motor neurons innervate the circular and longitudinal muscle layers. Excitatory motor neurons synthesize acetylcholine, which they release from their varicose endings in the smooth muscle layers; some also release the tachykinin peptides, substance P and neurokinin A, which excite smooth muscle. Typically, axons of excitatory motor neurons project either directly to the smooth muscle close to their cell bodies or orad for up to 10 mm.4 Once in the smooth muscle layers, the axons turn and run parallel to the smooth muscle fibers for several millimeters; they branch extensively and form many small varicosities, or transmitter release sites, closely associated with intramuscular ICC (ICCIM).
Inhibitory motor neurons typically are slightly larger than excitatory motor neurons, and there are fewer of them. They also have short dendrites and a single axon but, unlike excitatory motor neurons, they project aborally to the smooth muscle layer for distances of 1 to 15 mm in the human colon.4 Once the axon reaches the smooth muscle, it branches extensively to form multiple varicose release sites. Inhibitory motor neurons release a cocktail of transmitters that inhibit smooth muscle cells, including nitric oxide, adenosine triphosphate (ATP), and peptides, such as vasoactive intestinal polypeptide (VIP) and pituitary adenyl cyclase-activating peptide (PACAP). The varicose transmitter release sites of inhibitory motor neurons also are associated with ICCIM, just as are the release sites of excitatory motor neurons. Interstitial cells probably mediate a large component of the electrical effects on smooth muscle of neurotransmitters released by enteric motor neurons. Inhibitory motor neurons usually are tonically active, modulating the ongoing contractile activity of the colonic circular smooth muscle. Inhibitory motor neurons are particularly important in relaxing sphincteric muscles in the ileocecal junction and the internal anal sphincter.
SYMPATHETIC INNERVATION
The major sympathetic innervation of the proximal colon arises from the inferior mesenteric ganglion and projects through the lumbar colonic nerves to the ascending and transverse colon (see Fig. 98-3). A small number of sympathetic neurons in the celiac and superior mesenteric ganglia, in the paravertebral chain ganglia, and in the pelvic plexus ganglia also project to the colon (see Fig. 98-3). These neurons receive a powerful cholinergic drive from preganglionic nerve cell bodies in the intermediolateral column of the spinal cord (segments L2-L5). This is a major pathway by which the central nervous system modifies bowel activity, such as during exercise. Sympathetic efferent neurons also receive input from the enteric viscerofugal neurons and from extrinsic, spinal sensory neurons with cell bodies in the dorsal root ganglia, forming several reflex loops with the distal bowel.