Fig. 34.1
Average time (in hours) from injury to arrival at a field hospital for major American conflicts (GWOT global war on terror) (Modified with permission from Perry et al. [4] © in 2004 Elsevier)
The historical trends and advancements in the management of colorectal trauma can largely be grouped around major conflicts and wars, when high volumes of penetrating abdominal wounds provided a large body of experiential data. As described in a historical analysis by V.J. Cirillo, the epidemiology of battlefield wounds and deaths can be roughly divided into two eras with World War I as the dividing line [5, 6]. Prior to and leading into World War I (Disease Era: 1775–1918), the most common causes of battlefield morbidity and mortality were not wounds or injuries, they were communicable disease, infection, and hypothermia. This epidemiology rapidly changed with World War II (Trauma Era: 1941–present), where improvements in medical care drastically reduced deaths due to disease and the development of modern weaponry heralded the rise of trauma as the primary cause of battlefield deaths.
Prior to World War I, the management of abdominal wounds was largely nonoperative, and the mortality approached 100 % if a bowel injury was present. Laparotomy was largely condemned as a treatment option, as summarized by MacCormac’s experience from the Boer War (1899–1902) where he stated that “In this war a man wounded in the abdomen dies if he is operated upon and remains alive if he is left in peace” [7]. However, in the presence of evisceration with relatively easy access to the injured bowel, it was recognized that primary repair and return of the bowel to the abdominal cavity was advantageous [4]. With the understanding that repair of these injuries was clearly superior to observation alone, it was mainly a matter of waiting for the development of equipment and medications that would allow for safe anesthesia and postoperative recovery.
During World War I, the sheer volume of penetrating abdominal wounds coupled with advancements in casualty evacuation and prehospital trauma care led some surgeons to abandon nonoperative management in favor of laparotomy and primary repair of the injured colon [8, 9]. Although this approach was still associated with a considerable risk of repair failure, infection or sepsis, and death, there was a notable decrease in the overall battlefield mortality and particularly the mortality associated with abdominal wounds [10, 11]. During World War II, the next major paradigm shift in colorectal trauma management followed the publication of Ogilvie’s classic analysis of the management of colon wounds from the North African campaign of 1942 [12]. He strongly advocated the use of fecal diversion for all colon injuries by either exteriorization of the wounded area of the colon or repair/resection with proximal diversion and credited this approach with the drastically reduced mortality rates compared to WWI [12, 13]. This approach became the formal doctrinal policy for surgeons of both the British and US medical forces, and proximal diversion was mandated for treatment of all colon wounds by the US Surgeon General in 1943 [13, 14]. For rectal injuries, the high risk of mortality from pelvic and retroperitoneal sepsis was significantly decreased during WWII by adopting the principles of wide local washout/debridement in addition to proximal diversion [4, 15]. This was most commonly accomplished by a posterior approach with excision of the coccyx to access the presacral space for washout and drain placement [15].
Further advances in the management of colorectal trauma over the next several decades arose from advances in surgical technique as well as improvements in prehospital care and evacuation during combat operations. Surgeons of the Korean and Vietnam War eras adopted a more anatomic-based approach to colon injuries, favoring resection and primary anastomosis for select right-sided injuries and exteriorization and/or colostomy for left-sided or rectal injuries [4]. One series from the Vietnam conflict reported excellent results with the use of a “closed colostomy” for isolated colon injuries, with primary repair of the injury followed by exteriorization of the repaired segment to observe for any postoperative suture line breakdown [16]. However, other series reported significantly worse outcomes with this approach, and exteriorization has been largely abandoned in favor of alternative techniques. For rectal trauma, the addition of distal washout of the rectum was reported to decrease infectious complications and completed the well-known “4 Ds” of rectal trauma: direct repair, divert, drain, and distal washout.
The prolonged peacetime experiences following the Vietnam War resulted in further refinements of management principles and operative techniques for colorectal trauma that are discussed in this chapter. This has also been the most productive time period in terms of generating controlled data on which to make evidence-based management decisions. In addition to retrospective case series, a number of higher-quality studies including prospective series, case–control studies, multicenter studies, and even randomized trials have been reported and have helped to drive further improvements in outcomes from colorectal injury. The overall trend of the past two decades can be summarized by these six principles:
1.
Primary repair for most nondestructive, low-velocity type injuries.
2.
Resection with anastomosis for most destructive and/or high-velocity injuries.
3.
Elimination of routine proximal diversion for most colon injuries and select rectal injuries.
4.
Decreased emphasis on anatomic location of injury as an important factor in management for colon injuries.
5.
Adoption of damage control laparotomy principles allows for delay of decision making regarding diversion versus anastomosis in unstable or high-risk patients.
6.
Tailored management of rectal injuries with individualized use of the “4 Ds,” with proximal diversion remaining the most common and important.
Most recently, there have been several excellent series detailing the experience with colorectal trauma in the conflicts in Iraq and Afghanistan over the past decade [2, 3, 17]. These series demonstrate that the trends observed in civilian practice of extending primary anastomosis without diversion to colon trauma have carried over to the military setting, with the majority of colon wounds being managed with primary repair or resection and anastomosis [2]. However, approximately one-third of patients were still managed with a diverting colostomy, demonstrating that proximal diversion remains an important and frequently used component in the arsenal of the combat surgeon. Analysis of these patients’ longer-term outcomes demonstrated the viability of primary repair or anastomosis, but also highlighted the higher risk of repair failure in the presence of associated abdominal injuries such as pancreas, stomach, spleen, diaphragm, and kidney [17]. With the realization that better data collection and analysis is critical to optimizing combat research, a dedicated Joint Surgical Transcolonic Injury or Ostomy Multi-Theater Assessment (J-STOMA) project and database has been established. The first published series of 977 patients from the J-STOMA database has provided valuable epidemiologic data and also identified a significant difference in mortality between patients with no fecal diversion (11 %) versus 4 % with fecal diversion [18].
A unique factor that also must be considered is that this is the first major combat experience where damage control principles have been widely adopted and utilized [19–21]. The adoption of damage control laparotomy techniques allows for a delay in the decision regarding anastomosis versus proximal diversion in the unstable or high-risk patient, which can then be performed at a later time in a more elective setting. Preliminary follow-up data from primary repair or anastomoses performed in the combat damage control setting have demonstrated acceptable morbidity rates and outcomes comparable to civilian patients undergoing damage control laparotomy [17, 19].
The radical overall change in the management of colorectal trauma is highlighted by the findings of a 1998 survey of US trauma surgeons regarding the management of these injuries [22]. The overwhelming majority (98 %) would use primary repair or resection with anastomosis for selected colon injuries, and almost one-third of respondents stated that they would “never” perform a colostomy for colon trauma. This stands in stark contrast to the World War II dictum of mandatory colostomy or exteriorization for all injuries. Although this is generally accepted to represent clear progress in the management of colorectal trauma, there is continued debate about whether the pendulum has swung too far in favor of primary anastomosis, particularly for war-related injuries [23]. Further controlled trials and reports of larger experiences with good long-term outcome data will undoubtedly help us to further clarify the optimal management techniques in both civilian and military settings.
Colon Trauma
Key Concept: Trauma surgery is all about rapid evaluation and prioritizing everything you do – starting from your physical exam and including resuscitation, imaging, and operative interventions.
Key Concept: Controlling life-threatening hemorrhage and stopping GI tract contamination are the top two priorities in abdominal trauma – and major bowel injuries often present with both. Failure to rapidly diagnose and intervene is lethal.
Many of the details of the surgical anatomy and techniques for various colorectal procedures are discussed in great detail by expert surgeons in other chapters of this text. Although there may certainly be many complicating factors such as anatomic distortion, poor visualization due to bleeding, and multiple simultaneous injuries in the trauma patient, the basic steps and technical considerations of the operation are no different than in most elective settings. The critical differences in successfully managing colorectal injuries are in the thought process and decision making – both preoperatively and in the operating room – that often need to be made rapidly with imperfect and incomplete information, in suboptimal and often chaotic settings, and under the pressure of time constraints (i.e., the “golden hour”). Therefore, this chapter will focus more on the key decision-making processes, common pitfalls, and optimal management strategies and less on the procedural details or surgical technique.
The fastest way to fail at managing the severely injured patient is to approach them with an elective surgical mindset. In the elective setting the priority is placed on being thorough and taking a “head to toe” systematic approach to evaluation in order to identify all pertinent medical and surgical issues. In the trauma setting, the exact opposite is true. A rapid, focused, and highly prioritized evaluation should be performed with the initial focus only on identifying potentially life- or limb-threatening injuries along with select and highly relevant aspects of the patient history. Attention can often be distracted by more dramatic but non-life-threatening injuries (i.e., open extremity fracture), and although it is difficult, these should be initially ignored (other than active hemorrhage) until completion of the primary survey and truncal exam. With respect to colorectal injury, the key aspects of the initial evaluation are shown in Table 34.1. This evaluation should focus on both identifying the presence of a colorectal injury as well as providing the key patient-related information that may alter your management decisions and risk: benefit analysis for primary repair versus diversion or another alternative.
Table 34.1
Key elements of the initial trauma evaluation for colorectal trauma
History | Physical examination | Diagnostic and imaging studies |
|---|---|---|
Abdominal pain or complaints | Overall impression (“sick” or “not sick”) | Chest x-ray – free air, elevated or blurred diaphragm |
Allergies and medications | Vital signs | FAST exam – free fluid in abdomen or pelvis |
Prior abdominal surgery: particularly any prior bowel surgery, hernia repairs, mesh implantation, aortoiliac surgery | Focused abdominal exam: tenderness, distension, rebound, guarding, bruising, “seat-belt sign.” Identify all prior incisions, any hernias | CT scan abdomen/pelvic: diagnostic study of choice in most patients. No oral contrast required for initial study. Consider follow-up CT with oral contrast or “triple contrast” for equivocal initial study or concerning clinical picture |
Major comorbidities: vasculopathy, congestive heart failure, high-dose steroid use, immunosuppressants | Location of all open or penetrating wounds | “Triple-contrast” CT scan: may be useful for penetrating flank or back wounds with suspicion for retroperitoneal colon injury, but usually standard CT is adequate |
Injury mechanism (from high to low risk) | Logroll and full back/flank exam | Abdominal x-rays: not useful as routine study in blunt trauma. Can be very useful in gunshot wounds for identifying location of fragments, estimating trajectories. Place radiolucent markers on all external wounds |
Penetrating, missile | ||
Penetrating, stab | ||
Blunt, high velocity | ||
Blunt, low velocity | ||
Pelvic and perineal exam | Diagnostic peritoneal lavage: mainly of historical interest, but can be used with equivocal CT findings (i.e., free fluid with no solid organ injury) in patients with unreliable exam | |
Digital rectal exam (DRE) | Anoscopy, rigid proctoscopy: penetrating perineal trauma, open pelvic fracture, positive DRE, any other suspicion for rectal injury |
Injury mechanism and location can be extremely helpful for gauging your level of suspicion of a bowel injury and for directing further workup. The highest level of suspicion should be for gunshot wounds to the trunk, particularly those that have clearly passed from anterior to posterior or crossed the midline from side to side. The default position for these wounds should be to assume that bowel injury is present and to perform a laparotomy. Perineal or trans-pelvic gunshot wounds and those with an associated pelvic fracture should be assumed to have a rectal injury until proven otherwise. Stab wounds to the abdomen, flank, back, or perineum carry a lower but still significant risk of colorectal injury and warrant a detailed exam, and often supplemental imaging, unless there are obvious signs of a bowel injury at presentation. The old approach of exploring the stab wound and performing a laparotomy if there is evidence of fascial penetration has largely been replaced with decision making based on the abdominal exam and use of imaging studies [24, 25]. For blunt trauma, the incidence of colorectal injury is significantly lower and much less common; therefore, it can be frequently misdiagnosed or overlooked completely. High-risk mechanisms are those that involve high velocity or forces of impact and include motorized vehicle crashes, falls from heights, or pedestrians struck by vehicles. Patients with lower mechanisms of blunt trauma such as falls from standing, low-speed motor vehicle collisions, or non-motorized vehicle crashes have exceedingly low risk of colon or rectal injuries and usually do not require additional studies if the history and exam is reassuring. A particular warning should be made for patients with a high-risk mechanism and an altered exam, typically from brain injury or due to alcohol or drug use. Supplemental imaging, usually with a CT scan, should be the standard, and a lower threshold for exploratory surgery should be maintained.
“Catatonia” and Early Decision Making
Key Concept: “I thought I would get a quick CT scan to rule out other injuries” is a commonly heard refrain at M&M conferences when explaining why a patient with operative abdominal injuries died in the CT scanner.
The sharp decline in civilian penetrating trauma volume and the increase in nonoperative management of most injuries have inadvertently created a widespread disease pathology among surgeons and surgical trainees that we have previously described as catatonia [26]. Defined as “the inability to make definitive management decisions without the use of detailed computed tomography imaging, coupled with a fear of the exploratory operation,” this is a frequent cause of unnecessary delay to definitive operative intervention and a cause of preventable morbidity or mortality. An oft-heard excuse when explaining a major delay in operative intervention for a perforated colon is that the surgeon wanted “more information” about the injury or that the patient “needed resuscitation” before going to the operating room. In this chapter we emphasize the “golden rule” of the acute abdomen; any patient with an acute abdomen or peritonitis, or obvious signs of a bowel injury on initial exam or plain films, belongs in the operating room immediately. No prolonged imaging workup is required, and resuscitation can be done just as well in the operating room as it can anywhere else in the hospital. In the stable patient this approaches may save you time and resources; in the unstable or deteriorating patient with a colorectal injury, these approaches may save his or her life.
In the Operating Room
Once the decision has been made for operative intervention for a known or suspected colorectal injury, all of the basic principles of emergency surgery and ATLS apply. Although attention should be paid to hypothermia prevention and management with active warming devices (heated forced-air blanket), one exception to common practice that we believe should be omitted is the warming of the operating room to excessive ambient temperatures (>75 °F). This has been shown to be wholly ineffective at treating or preventing hypothermia and only results in discomfort of the operative team and likely additional contamination of the operative field due to sweat [27]. All patients should be expected to have a full stomach, so aspiration precautions during rapid sequence intubation are critical. Bleeding and ongoing blood loss should be anticipated, so emergency-release blood products (type O for packed cells and type AB for plasma) should be standing by and a type and cross should be performed as soon as possible. A Foley catheter should be placed barring any signs of urethral injury, although if there is a suspicion for bladder injury, then we recommend prepping in the genitals and placing a sterile catheter that can be accessed by the surgeon. This can be invaluable for manually distending the bladder with fluid and/or dye to identify injuries and to test for leaks after a repair.
It is important to optimize the operative exposure and visualization, and a few minutes to set up a self-retaining retractor (Bookwalter, Omni, etc.) will pay dividends in decreased overall operative times and frustration levels. This is particularly important for difficult exposure situations such as obesity or for pelvic dissections (sigmoid, rectum). This will also free the hands of the surgeon and assistant to perform more critical tasks. For the unstable or actively bleeding patient, a generous midline incision should be made at the start to allow rapid access to all quadrants of the abdominal cavity. For the stable patient, a smaller laparotomy incision may be made and then extended if necessary based on the injuries identified. Most right-sided colon injuries can be managed through a supra-umbilical midline incision, but left-sided or rectal injuries require inferior extension below the umbilicus toward the pubic symphysis. Descending or sigmoid colon injuries that require resection with anastomosis typically require the longest incisions to adequately mobilize the left colon (including the splenic flexure) and then perform an anastomosis in the lower abdomen or pelvis. If a large amount of hemoperitoneum is discovered on entering the abdominal cavity (even in a “stable” patient), then abandon any attempt at “minilaparotomy” and widely extend the incision from the xiphoid to several centimeters above the pubic symphysis.
Prepped Versus Unprepped Bowel
One of the most important factors that led to a revolution in the performance of colorectal surgery was the ability to decrease the risk of postoperative infectious complications. The high bacterial content of the colon has long been recognized as an area of concern with any intraoperative spillage, and the concept of increasing bacterial concentrations in the distal colon was a principal factor in promulgating the belief that left-sided injuries should be treated differently than right-sided injuries. For elective surgery, the standard of care has traditionally been to administer an oral mechanical bowel prep (with or without oral antibiotics) before surgery to both remove fecal material and to decrease the bacterial load in the event of intraoperative spillage [28]. In cases of emergent trauma with colorectal injury, the obvious lack of bowel prep before injury has been cited as one reason to treat these differently than elective operations and to forego primary repair or anastomosis in favor of diversion. Over the past decade, a large body of high-quality evidence has amassed that refutes the standard beliefs about the value of mechanical bowel preparation, with multiple randomized trials and several meta-analyses finding no benefit or even evidence of increased morbidity with bowel prep [29, 30].
The currently available data has led many surgeons to abandon or to more selectively utilize bowel prep for elective surgery, but it also provides reassurance for trauma surgeons that a preoperative bowel prep is not a requirement for a safe bowel repair or anastomosis. The two primary issues that a classic bowel prep was designed to address are (1) mechanical removal of fecal matter and (2) administration of antibiotic prophylaxis against infections due to fecal flora. For the vast majority of traumatic colorectal injuries, mechanical clearance of the colon is unnecessary and in fact would only serve to delay completion of the procedure. The second goal of infection prophylaxis is achieved with the administration of intravenous antibiotics that cover both aerobic and anaerobic organisms typical of fecal flora. Antibiotics should be administered as soon as there is evidence of the injury or a decision for laparotomy has been made. Although the optimal goal would be 30–60 min prior to the skin incision, this is often not realistic in trauma surgery. Re-dosing of antibiotics should be performed if the surgery is prolonged to near the normal dosing interval for the selected antibiotic or in cases where blood loss and transfusion are approaching one whole blood volume. Antibiotics should be continued for 24 h only, and there is no benefit (and potential harm) of continuing them longer even in the face of large volume contamination [31]. Appropriate single-agent therapy is as effective as multidrug regimens, and most centers use a B-lactam agent such as Unasyn or Zosyn. In class 4 cases with a delayed presentation and established infection, antibiotics should be continued as dictated by the location and nature of the infection.
Finally, an additional well-described option that can be utilized is the “on-table” bowel preparation or lavage [32]. This concept was initially espoused for all emergent colon surgery in order to achieve the same benefits as a standard preoperative bowel prep. However, it has fallen out of favor as the role of mechanical bowel preparation has been questioned and due to the difficulty of adequately lavaging the colon effectively and without spillage. The pros of an on-table lavage include the ability to debulk and clean the colon to facilitate easier repair or anastomosis, decompress a dilated colon, and remove a large stool burden that could cause future constipation or impaction. The cons include the lack of a proven benefit, the possibility of increased spillage and infections, and the need for making additional holes in the colon or small bowel to place the lavage catheter. We recommend consideration of on-table lavage only in situations of major colonic distension with a large amount of solid or liquid matter or significant solid fecal impaction that could impair recovery of motility or anastomotic healing. Some technical tips/tricks include as follows: (1) lavage the entire colon by opening the appendix and inserting a balloon-tipped catheter for instilling the fluid, then perform a standard appendectomy after catheter removal, and (2) insert a sterile ventilator tubing into the open proximal end of the colon to be lavaged and secure it with a large suture or umbilical tape (Fig. 34.2).
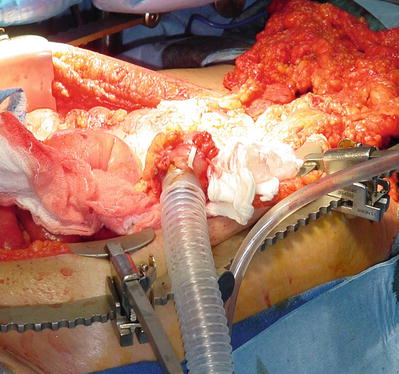

Fig. 34.2
On-table lavage of distal colon and rectum is performed using sterile ventilator tubing placed in the open end of the distal colon and secured with an umbilical tape
Also remember to use warmed fluids for the lavage in order to avoid causing or exacerbating systemic hypothermia.
Timing of Injury and Operative Decisions
Key Concept: Most traumatic colorectal injuries will present within the first few hours and a short delay should not impact management decisions. Longer delays with uncontrolled abdominal contamination should push you toward diversion and/or damage control techniques.
Key Concept: Delayed diagnosis of a contained injury with minimal associated symptoms should prompt consideration of nonoperative management – if the patient has already passed this “trial of life,” then CLOSE observation or percutaneous drainage may be all they need.
One of the most important factors in determining the optimal approach to a colorectal injury is the duration of time that has elapsed between the initial injury, recognition of the injury, and intervention for the injury. Fortunately, for the vast majority of traumatic injuries to the colon or rectum, patients are immediately transported to a trauma hospital and the diagnosis is established on the initial evaluation and imaging. It also appears that minor delays in diagnosis or intervention (2–8 h) do not have much impact on postoperative patient outcomes, and this would typically not change any intraoperative decision making. This also applies to patients that present in a delayed fashion due to a partial-thickness injury that then progresses to full-thickness injury with perforation. As long as the change in status is quickly identified and intervention performed in a timely manner, there does not appear to be major added morbidity or mortality.
However, occasionally there may be a significant delay to either diagnose or intervene in a patient with a major colorectal injury, and this can result in severe morbidity or even mortality. Scenarios where this may occur include failure to recognize peritoneal signs or imaging findings at initial presentation, presence of factors that compromise the abdominal exam (such as severe head injury or intoxication), patient delay in seeking medical attention or in transfer to an appropriate facility, and masking of peritoneal signs by medications (i.e., steroids) or other patient factors. Delays of more than 8–12 h associated with fecal contamination will typically significantly alter both the local anatomy and the patient physiology, and as a result, the intraoperative decision making and choice of procedure will frequently have to be adapted based on the findings.
As a general principal, there should be a much more liberal use of proximal diversion rather than primary anastomosis or repair when there has been a long delay to operation in the setting of fecal contamination and peritonitis. Some of the key considerations and technical factors that may alter your approach in these cases include:
1.
Prolonged fecal contamination can result in a SIRS response or even septic shock, and the patient may not tolerate prolonged surgery or reconstruction.
2.
Pelvic sepsis after extraperitoneal rectal injuries can be rapidly fatal and may have little to no visible intra-abdominal pathology.
3.
Bowel wall induration and edema may compromise staple or suture lines.
4.
Massive small and/or large bowel distension may be present.
5.
The mesentery is often thickened and shortened, limiting mobility and making even a simple colostomy difficult or impossible.
In select cases the patient may present with delayed recognition of a colorectal injury and minimal or localized associated signs or symptoms. In this case the patient has already demonstrated tolerance or control of the injury, and temporizing minimally invasive interventions may be all that are required. The classic non-trauma example of this would be perforated diverticulitis or appendicitis with a localized abscess that can be managed with antibiotics and a percutaneous drain. This is infrequently encountered in the trauma setting but may be seen more frequently with iatrogenic colorectal injury from endoscopy, biopsy, or other interventions. In these cases the optimal management strategy is usually to fully characterize the pathology with a high-quality CT scan, cover the patient with intravenous antibiotics, initiate bowel rest and NPO status, and ensure drainage of significant abscesses or fluid collections. This most often can be accomplished with image-guided percutaneous or transrectal drainage but may require laparoscopy or even laparotomy for difficult locations. Percutaneous drain placement may also be considered in the patient with a large amount of free air who is otherwise a good candidate for nonoperative management. Aspiration of the free air with or without placement of a drain can markedly improve the abdominal discomfort and provide an improved baseline for serial physical examinations.
Operative Management: Repair, Resect, or Divert?
Key Concept: Injury location used to drive management decisions for colon trauma, but this distinction has largely been abandoned and management should be based on the exact colon injury, associated injuries, and patient factors.
Key Concept: Remember to consider ALL of the risks for repair versus diversion, including the morbidity associated with a second surgery for ostomy takedown. This risk analysis should most often favor primary repair or anastomosis, but a diverting or protective ostomy still has a clear role in high-risk injuries or patients.
Once a full exploration has been completed and all bowel injuries identified, the first decision is whether the injury is amenable to primary repair or will require segmental or formal anatomic resection. The standard teaching is to categorize injuries as either destructive (>50 % of bowel circumference or devascularized) or nondestructive, with resection recommended for destructive and primary repair for nondestructive wounds. Although this is a good basic guideline, there are several other important factors to consider and these are outlined in Table 34.2. These include not only the size of the injury but the number and location of injuries as well as the adequacy of primary repair that may be achieved. An important technical point for missile wounds, particularly fragment or high-velocity gunshot wounds, is that the wound edges need to be debrided back to healthy tissue before closure. These missiles will frequently cause extensive tissue damage or even direct thermal injury (Fig. 34.3) and will often break down within several days of closure if not adequately debrided. Thus, a small wound that initially appears appropriate for primary repair may actually require segmental resection once the full extent of tissue injury is identified and debrided. On the other hand, excessive debridement of surrounding tissue for high-velocity injuries has been advocated based on erroneous assumptions about the size and impact of the “temporary cavity” created by the missile. In addition to these overestimates of the size and force of cavitation, it is important to note that elastic tissues such as bowel tolerate stretch forces much better than inelastic tissue such as the liver [33]. Debridement of all clearly devitalized and any questionable tissue should be performed, but do not perform additional debridement of healthy tissues simply based on the injury mechanism or ballistics. The status of the mesentery is also an important factor as it relates to the adequacy of the blood supply to the injured area. A classically described injury seen in blunt trauma with rapid deceleration is a large tear of the mesentery without injury to the colon wall. This “bucket-handle” deformity can be misleading as the bowel will usually appear uninjured but should be resected due to the large area of devascularization (Fig. 34.4).
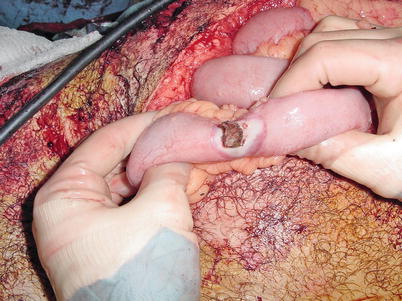
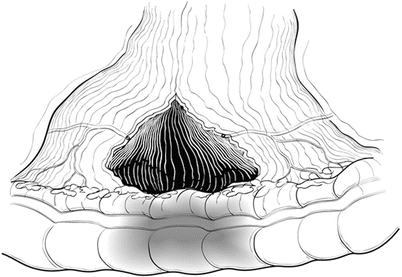
Table 34.2
Factors to guide primary repair versus resection for colon injury
Primary repair | Resection |
|---|---|
Small size (nondestructive) | Destructive (>50 % circumference or devascularized) |
Single injuries or multiple with adequate spacing | Multiple injuries with short spacing |
Clean margins (after debridement of edges) | Inflamed or necrotic edges |
Minimal or no mesenteric injury | Large mesenteric hematoma or laceration |
Tension-free closure | Cannot be closed without tension |
Healthy surrounding bowel | Major edema, inflammation, bowel wall hematoma |
No major pathology present | Major pathology present (cancer, diverticulitis, etc.) |
Closure leaves widely patent lumen | Closure would narrow lumen (>25 %) |
Low velocity wound | High-velocity wound |
At risk for short-gut syndrome with resection | Adequate bowel length after resection |
No adjacent pancreatic injury or leak | Pancreatic injury/leak adjacent to injury |

Fig. 34.3
Missile wound to bowel with small perforation but significant thermal injury to the surrounding bowel wall. This injury should be completely debrided and then repaired or resected

Fig. 34.4
Large tear of the mesenteric border of the bowel (“bucket-handle” deformity) from blunt deceleration forces. This usually requires resection of the now devascularized bowel segment to avoid subsequent ischemic complications (Reprinted with permission Martin and Beekley [26] © in 2011 Springer)
As discussed above in the historical overview of colorectal trauma, a dogma developed over time of categorizing the wounds into “right”- or “left”-sided injuries and basing the management off of this simple distinction. Right-sided injuries could undergo standard repair or resection with anastomosis, but left-sided injuries required exteriorization or proximal diversion. This was based on microbiological data showing increasing bacterial loads in the left colon and the observation of increased complications with left sided or more distal colon injuries. However, subsequent experience with both traumatic colon resections as well as emergent resection for diverticular disease demonstrated that left-sided repair or resection with primary anastomoses could be performed with an acceptably low risk of anastomotic leak or other major morbidity.
A major potential confounder of studies examining the question of right- versus left-sided colon injuries is the unequal distribution and severity of associated injuries. The right colon generally lies outside of the pelvis and is not intimately associated with any major vessels or organs except for the right kidney. Alternatively, the transverse colon lies over the body of the pancreas and the aorta/vena cava, while the sigmoid colon lies in the pelvis adjacent to the iliac vessels, bladder, ureter, sacral veins, and pelvic bones. Thus, left-sided colon injuries are much more likely to have associated vascular, pancreatic, and genitourinary injuries or a major pelvic fracture. The presence of these associated injuries will naturally increase the risk of blood loss, need for transfusion, duration of surgery, and systemic inflammatory response. As a result, morbidity and mortality is higher among this patient cohort, but most of this increase is likely independent of the colon injury. Some other key technical factors also play a likely role, including the difficulty of working in the narrower pelvis (left sided) versus the mid to upper abdomen (right sided) and the higher likelihood of anastomotic tension or ischemia with a colorectal anastomosis versus an ileocolic anastomosis.
Although still occasionally taught, the trauma community has largely abandoned the management of colon injuries according to the simplified right-left dichotomy. However, there is now a commonly held misunderstanding that the injury location is not important and that all colon injuries can be treated in a similar fashion regardless of location. Although the left-right distinction is no longer of primary importance, it is critical for the surgeon to appreciate the key issues and technical factors that will be encountered in different injury locations. Table 34.3 shows some of these important considerations according to the exact area of injury from the cecum to the rectum. Each of these individual colon and rectal injury types and locations has an associated “textbook” answer for what operation should be performed, but these do not take into account the wide variations in disease presentation, presence of multiple bowel injuries, presence of associated injuries, and the patient physiology at the time of operation. There are multiple standard and nonstandard surgical options that can be used alone or in combination, and it is critical to tailor your approach primarily to the patient and not only to the disease. We recommend a surgical approach based on the following general categorizations of the patient and the injuries present:
Table 34.3
Key considerations and patterns of associated injuries based on the location of the colon injury
Injury location | Key considerations and associated injuries |
|---|---|
Cecum/ascending colon | Easiest area to mobilize and can be done rapidly with blunt hand dissection |
Assess for associated injury to right kidney and proximal ureter | |
Large perinephric or retroperitoneal hematoma can displace ureter much more medially or anteriorly than normal | |
Hepatic flexure | Frequently associated injury to liver that is usually obvious |
Also carefully assess gallbladder and porta hepatis | |
Diaphragm injuries frequently missed – remember to look and palpate over the dome | |
Always inspect the duodenum and head of pancreas! | |
Transverse colon | If associated zone 1 retroperitoneal hematoma, worry about the aorta and vena cava first |
Always open the gastrocolic ligament widely as this aides mobilization but also exposes: | |
(a) The posterior surface of the stomach – frequent site of missed gastric injuries | |
(b) The body and tail of the pancreas – critical to drain or resect any injury, particularly if you are doing an adjacent colon anastomosis or primary repair | |
(c) Posterior surface of the transverse colon mesentery | |
Injuries at the root of the mesentery may involve the SMA/SMV, middle colics, or D4 | |
Splenic flexure | May have a true lienocolic ligament or may be closely adherent to inferior pole of spleen |
Associated splenic injury common and usually obvious with hematoma or active bleeding | |
If the hilar area of the spleen is involved, then carefully look for an injury to the pancreatic tail | |
Diaphragm injuries frequently missed – look and palpate with spleen retracted inferomedially | |
Descending colon | Relatively easy to mobilize – sharply incise white line and the rest can be done bluntly |
Look for an associated zone 2 retroperitoneal hematoma = kidney or renovascular injury | |
Assess for injury to proximal left ureter; hematomas can displace ureter as stated above | |
Sigmoid colon | Transition from abdominal cavity to the true pelvis – multiple critical structures in a small space |
Always identify ureter and assess for injury; drain all identified or suspected injuries | |
Zone 3 hematoma = (1) pelvic fracture if blunt trauma or (2) iliac vessel injury if penetrating | |
If you have any suspicion for pelvic vascular injury, get proximal control at distal aorta/cava first | |
Rectum | Can be injured by blunt shear forces or penetrating missiles, but also bone fragments from pelvic fractures – have a low threshold for proctoscopy with major pelvic fractures Intraperitoneal rectum should be treated like sigmoid colon injury |
Associated injuries to all pelvic structures should be considered, including distal ureters, iliac arteries and veins, bladder, uterus/vagina, prostate, spermatic cord | |
Intravenous methylene blue can be helpful if injury to ureter is unclear | |
Any gross hematuria is a bladder injury; placing a sterile Foley catheter can be helpful for inflating the bladder to identify an injury or after a bladder repair to test for leaks | |
Of the “4 Ds” for rectal injuries, diversion is the most important in the majority of cases |
1.
Low Risk: Surgical intervention on a stable patient, adequately resuscitated and physiology either normal or steadily improving, minimal to moderate contamination, no major associated injuries, and medically fit for surgery with no high-risk factors or major systemic disease
2.
Moderate Risk: Immediate surgical intervention required due to patient instability, bleeding, or peritonitis. Moderate to large contamination, associated injuries but not immediately life threatening, hemorrhage controlled, non-coagulopathic, responding appropriately to resuscitation, and base deficit elevated (>5) but decreasing with resuscitation. Presence of comorbid disease, but medically controlled
3.
High Risk: Emergent surgical intervention required and has >1 risk factor including ongoing large volume hemorrhage, receiving massive transfusion, coagulopathic (INR > 1.5), metabolic acidosis (base deficit > 5), large contamination, delayed diagnosis with fecal peritonitis, severe bowel edema, massive dilation, associated major vascular injury, or pancreatic injury. Presence of severe medical comorbid disease, high-dose steroid or other immunosuppressant therapy, elderly, active congestive heart failure, debilitated, malnutrition, and hostile abdomen
For low-risk patients, a standard primary repair or segmental resection should be performed, and continuity restored without the need for a colostomy or ileostomy. For moderate-risk patients, the majority can also safely undergo primary repair or resection with anastomosis and will not benefit from fecal diversion. In addition, placement of an ostomy will also subject them to the risks of a second surgery for ostomy takedown or the well-described possibility that they will never have their ostomy reversed [34]. A recent randomized trial in perforated diverticulitis demonstrated that only 58 % of end colostomies were eventually reversed [35]. For patients in the high-risk group, the decision for ostomy versus anastomosis can usually be deferred in favor of a damage control approach (see following section “Damage control laparotomy”). However, whether the decision is made initially or at a subsequent laparotomy, this is the patient population where a diverting ostomy should still be strongly considered. Although there is no level 1 evidence to support these decisions, a temporary diverting ostomy to allow both healing of the colon repair and recovery of the patient can be lifesaving. In addition, with more distal pelvic anastomoses (colorectal, ileorectal), the margin of error and tolerance to a leak is significantly decreased and could result in the need for a permanent ostomy. There are multiple intraoperative decisions and considerations that need to be made quickly and decisively, and that can have significant impact on both short- and long-term outcomes. Table 34.4 provides a partial list of these key decisions with associated factors and technical advice that should be considered.
Table 34.4
Key intraoperative management issues and decisions in colorectal trauma
Key decision | Factors to consider | Technical issues/pearls |
|---|---|---|
Primary repair or resection? | Size of injury Shape of injury (linear, round/stellate) Single or multiple Tissue quality Mesentery status (rents, hematomas, devascularized segment) | Debride injured or burned tissue Connect close injuries rather than leaving “bridges” Evacuate large mesenteric hematomas Close mesenteric tears Resect segment with “bucket-handle” mesenteric defect |
Damage control? | Patient stability Transfusion requirement Acid/base getting better or worse? Multiple injuries? Another reason for a “second-look” (i.e., borderline bowel viability) | Make decision early in case Proceed if patient improving, terminate if getting worse Vacuum-assisted temporary closure works best Usually no need for other drains |
Anastomosis or ostomy? | Patient baseline status (age, comorbidities, meds) Physiologic status Quality of the tissues Other injuries and proximity to anastomosis Body habitus, ability to properly site an ostomy | Consider difficulty and risk of ostomy takedown Be wary of anastomosis with an associated pancreatic injury! Obesity increases difficulty and complications with ostomy |
Anastomosis: hand-sewn or stapled? | Operative time Other injuries to address Personal experience and comfort Tissue quality, edema Anatomic area and bowel alignment Available equipment | No difference in leak or complication rates in most series Hand-sewn potentially more secure with suboptimal tissue quality, bowel wall edema Laparoscopic staplers great for pelvis, hard-to-reach areas or sharp angles |
Ostomy: loop, end, other? | High-risk anastomosis that needs protection? Need access to distal bowel segment? Body habitus Mesentery – shortened, edematous | Loop may reach skin easier with obesity or shortened mesentery May not get complete fecal diversion with a loop Remember the “end-loop” option (see text) Use an ostomy bar if any tension or obese patient Wrap ostomy in seprafilm for easier takedown |
Leave a drain? | No indication for routine drainage of bowel anastomoses Widely drain any other adjacent injuries (pancreas, bladder, etc.) Other reasons: associated abscess cavity, control ascites in cirrhotic patient | Avoid direct contact of drain with anastomosis larger sump drains usually not beneficial Make exit site remote from incision and any ostomy |
Place a feeding tube? | Degree of bowel injuries and surgery Estimated need for prolonged NPO status Estimated inability to take oral nutrition Need for feeding access as well as gastric decompression? Pancreatic or duodenal injury? | Generally avoid making additional holes in bowel in the trauma setting Stamm gastrostomy relatively safe and secure Higher complications with jejunostomy tubes with little benefit Consider intraoperative placement of nasojejunal tube |
If fecal diversion is felt to be indicated, then two additional (and in our opinion underutilized) options are available to the surgeon as a “compromise” solution between no diversion and standard ostomy placement. The first is to proceed with placement of a diverting ostomy with a plan for early reversal within 1–2 weeks of the initial surgery. This method allows for initial healing of the colon repair or anastomosis, and then a contrast study should be performed to demonstrate adequate healing without leak or stricture. This also allows time for the natural sorting-out process whereby patients will either progress appropriately following their trauma or develop additional complications or physiologic decline and thus eliminate them from consideration of an early ostomy reversal. A prospective randomized trial of this approach in patients with colon injuries demonstrated that early colostomy closure was safe and resulted in shorter operative times and less blood loss than delayed closure [36]. The second compromise option is to perform the definitive repair or anastomosis and then create a separate more proximal protective diverting colostomy or ileostomy. This concept has long been recognized in colorectal surgery for protection of low pelvic anastomoses or ileal pouch procedures and is also applicable to protecting the high-risk anastomosis in the trauma setting. The choice of the type and location of the ostomy should be mainly based on (1) completeness of fecal diversion, (2) ease of placement, and (3) ease of reversal. Other factors that should be considered include the body habitus and location of abdominal wall wounds and incisions that could alter the viable options available for siting the ostomy. Multiple studies have demonstrated that loop ostomies are clearly superior to end stomas in ease of placement and reversal and also provide adequate fecal diversion [36–38]. A recent prospective randomized trial in patients with feculent or purulent perforated diverticulitis demonstrated the superiority of this approach (primary colorectal anastomosis with protective loop ileostomy) versus a standard Hartmann’s procedure [35]. The protective loop ileostomy approach was proven safe and was associated with significantly reduced major complications, hospital stay, and costs. In addition, the choice of ileostomy versus colostomy appears to be equivalent in terms of the adequacy of diversion [38]. As the ileum is typically more mobile and of smaller caliber than the colon, a loop ileostomy is the technically easier option and provides more options for external siting. However, the surgeon must be cognizant that the risk of fluid and electrolyte problems due to high stoma output will be higher with ileostomy versus colostomy, and this can be particularly difficult to manage in elderly or debilitated patients. Our recommendation is that if a temporary protective ostomy is indicated, then a loop ileostomy (or end-loop ileostomy as shown in Fig. 34.5) is the best option and can easily be reversed without the need for laparotomy in most patients. An excellent alternative option, particularly in the patient who may need longer-term or even permanent diversion, is a loop transverse or sigmoid colostomy. Prior to ostomy reversal, all patients should undergo contrast imaging to document anastomotic healing and should have documentation of adequate anal sphincter function for fecal continence.
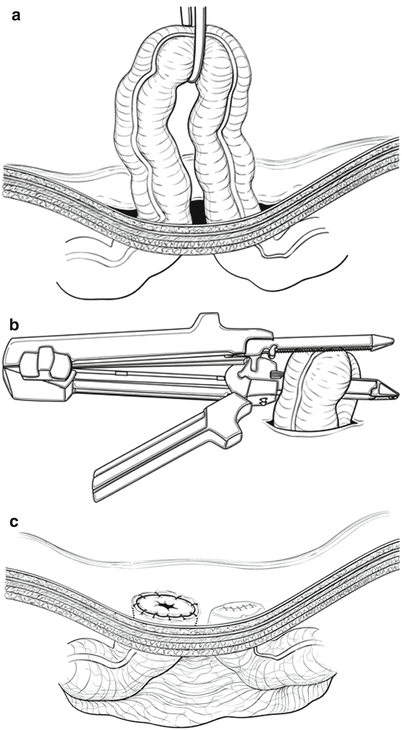

Fig. 34.5




Technique for end-loop ostomy (colon or ileum). (a) loop of bowel is delivered, (b) bowel is divided at the site of the planned ostomy, (c) proximal end is matured and the distal stapled end is secured in the subcutaneous position for easy future access and restoration of continuity (Reprinted with permission Martin and Beekley [26] © in 2011 Springer)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








