Kidney development in humans proceeds from the 9th to the 36th week of gestation.
9,
14 Accurate determination of nephron number is difficult because nephron number cannot be determined in humans in vivo. The unbiased fractionatorsampling/dissector method is thought to be the most objective nephron counting method, and is currently utilized in most human studies.
15,
16 This method, however, requires postmortem kidney samples and is very labor intensive. An in vivo glomerular counting method comparing the fractionator technique with a combined renal biopsy/magnetic resonance imaging (MRI) method in explanted canine kidneys has been attempted.
17 This study found a good correlation of glomerular number on average between the two methods, but, within kidneys, there was a 36% variance, calling individual applicability into question. Large-scale human
studies of nephron number and association with phenotype are therefore not easily feasible.
Average nephron number has been reported to range from 617,000 (range 331,000-1,424,000) to 1,429,200 (range 884,485-1,959,914) per kidney among normal adult Caucasian Europeans.
10,
18 Other studies including subjects of multiple ethnic origins from the United States, Africa, and Australia showed somewhat similar results, with a mean number around the mid 800,000 glomeruli per kidney, with a very wide range, from 210,332 to 2,702,079 as shown in
Table 2.1.
13 The range appears widest in kidneys from subjects of African origin.
13,
19 In general, nephron numbers are lower in older subjects, attributed to age-related glomerulosclerosis and obsolescence.
18,
20 Whether the high variability in nephron number across populations reflects true differences or is confounded by small sample sizes or limitations of counting methods will become clearer with time as more studies accumulate or as better techniques evolve.
Modifiable Factors
Modifiable factors associated with low nephron number include prenatal events—factors occurring during gestation and postnatal events occurring in the neonate.
Prenatal Factors. Maternal diets deficient in protein, total calories, or iron have all been shown to reduce nephron numbers in offspring of experimental animals, most often in association with low birth weight.
12,
23,
24,
25,
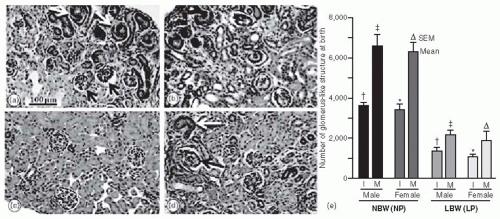
26 Figure 2.1 shows a reduction in nephron numbers in low birth weight rats that were subjected to maternal low protein diets during gestation. Maternal dietary deficiencies are common in pregnant mothers in developing countries and therefore likely clinically relevant in a large proportion of the world.
27 Maternal vitamin A deficient diets are associated with a dose-dependent reduction in nephron number in animals.
28 Vitamin A deficiency was examined in a cohort of Indian compared to Canadian mothers and found to be associated with significantly smaller newborn renal volume, which the authors suggest likely reflects lower nephron number.
29 Retinoic acid, the active metabolite of vitamin A, functions as a transcription factor regulating expression of Ret, a tyrosine kinase receptor critical for kidney development.
30 Interestingly, vitamin A levels are reduced by smoking and alcohol intake, both known to reduce birth weight.
31 Uteroplacental insufficiency, induced by uterine artery ligation late in gestation, also results in low offspring birth weight and low nephron number.
32,
33 This model may share some similarities with preeclampsia in humans in terms of the reduction of uterine blood flow and the restriction of fetal nutrient supply.
Increased fetal glucocorticoid exposure is a likely mechanism whereby maternal low protein diet reduces nephron number, via reduced activity of placental 11β-hydroxysteroid dehydrogenase activity, shown in both animals and humans.
34,
35 Similarly, administration of glucocorticoids during gestation in rats and sheep leads to reduced nephrogenesis, although this effect was not seen in the Marmoset monkey.
36,
37,
38 Glucocorticoids are thought to reduce nephron number by impacting ureteric bud invasion of the metanephric mesenchyme, thereby limiting branching morphogenesis.
8 The impact of maternal glucocorticoid utilization during pregnancy on human nephrogenesis is not known. Ingestion of other medications during pregnancy may also impact nephrogenesis in many ways.
39 Gestational administration of aminoglycosides, beta lactams, cyclosporine, cyclooxygenase inhibitors, and nonsteroidal anti-inflammatory drugs have all been associated with reduced nephron number in experimental models.
39,
40,
41,
42,
43 Similarly, chronic and acute gestational exposure to alcohol impairs embryonic ureteric bud branching, resulting in fewer nephrons in offspring.
44,
45 In humans one abstract suggested an impact of maternal alcohol consumption on kidney development in Australian Aboriginal children.
9Conceivably, therefore, all of these prenatal experimental conditions may impact human nephrogenesis and minimization of these exposures prior to and during pregnancy would optimize fetal nephrogenesis. The timing of an insult during gestation is also relevant to its impact on nephrogenesis, with the greatest effect in animals generally seen with interventions in the latter half of gestation.
8Maternal factors also impact fetal development during gestation. Low birth weight is associated with multiple maternal factors although nephron number has not specifically been examined in most cases.
46,
47 Manalich et al. found a strong correlation between low birth weight and low nephron number in a cohort of Cuban newborns.
48 Maternal hypertension and maternal smoking were correlated with low birth weight, although direct correlation with nephron number was not reported. In experimental animals, maternal diabetes or hyperglycemia has been shown to result in approximately 30% lower offspring nephron number in some, but not all, studies, although differences in methods of nephron number counting may account for some of the variability.
49,
50,
51 In other studies, maternal diabetes was associated with smaller kidneys, higher blood pressures, microalbuminuria, and reduced glomerular filtration rates in rat offspring.
51,
52 In young adults, renal functional reserve was found to be reduced in those who had been exposed to maternal diabetes during gestation, compared to those with paternal diabetes (i.e., excluding a genetic component), or those with nondiabetic parents.
53 The reduced renal functional reserve was interpreted by the authors as a possible surrogate for a reduced nephron number acquired in utero in the offspring of diabetic mothers.
Postnatal Factors. Although nephrogenesis is thought to be complete at birth in humans, this may not be the case for babies born prematurely, and therefore a window in which nephrogenesis may still be vulnerable likely exists soon after birth in these infants.
54 Consistent with this possibility, early postnatal growth restriction alone in normal birth weight rats was associated with a reduction in nephron number, demonstrating the importance of early postnatal nutrition on nephrogenesis.
32 The relevance of these findings to the human, however, is questionable because nephrogenesis normally proceeds for 10 days after birth in rodents and therefore this period is analogous to late gestation in humans. These data may, however, have relevance to humans born prematurely. Indeed, in a cohort of children born either very low birth weight (<1,000 g) or premature (<30 weeks gestation), extrauterine growth restriction was associated with significantly lower glomerular filtration rates at a mean of 7.6 years of age, suggesting an impact of postnatal nutrition on renal development.
55 Another study of postmortem kidneys from premature infants who died after 40 days of life found glomerular number to be significantly lower in those who developed renal failure compared to those who did not. These findings may suggest that renal failure itself inhibits glomerulogenesis; however, it is also possible that fewer glomeruli made these extremely ill infants more susceptible to renal failure. In another cohort of critically ill premature infants, renal failure was a significant complication and associated with a high mortality, although not associated with birth weight.
56 In contrast, another study did find neonatal acute kidney injury to be an independent predictor of mortality in very low birth weight infants.
57 Prematurity itself is a recognized risk for renal failure in infants, and has been shown to be associated with increased risk of subsequent hypertension and chronic kidney disease (CKD).
58 Taking these human studies together, postnatal events do impact renal development in premature infants and may have potentially adverse short- and long-term consequences.
Nonmodifiable Factors
Nonmodifiable factors also impact nephrogenesis, and may occur in isolation or together with other potentially modifiable factors described previously (
Table 2.2).
Genetics. Rare congenital and genetic abnormalities associated with abnormal kidney development manifest with renal dysfunction, often presenting very early in life.
11,
59 Approximately 40% to 60% of childhood end-stage renal disease (ESRD) results from some form of congenital renal hypoplasia.
60 More subtle renal developmental abnormalities—which may not manifest as overt syndromes but, rather, with later life renal dysfunction—may well be the result of gene polymorphisms impacting nephron number. Renal hypoplasia and reduced nephron number have been described with full or partial deletion of over 25 genes in mice, which are reviewed in detail elsewhere.
13,
21,
61 The important steps in kidney development
include specification of the metanephric blastema from the intermediate mesoderm, formation of the ureteric bud and its outgrowth from the wolffian duct, and ureteric bud branching. Genes participating in specification of the metanephric blastema from the intermediate mesoderm include
Odd-1, Eya 1 Pax 2, Wt-1, Six 1, Gdnf, and Sall 1, of which
Odd-1 and
Eya-1 are critical.
21,
60 Genes regulating formation of the ureteric bud and its outgrowth from the wolffian duct include
Pax2, Liml, Bmp4, and
Gdnf.21,
60 Gdnf (glial cell-derived neurotrophic factor) signals through the
Gfrα1 receptor and the
c-Ret receptor tyrosine kinase and, during branching, morphogenesis is only expressed on the tips of ureteric branches, selectively inducing branching at this location.
21 Among the most important pathways impacting nephrogenesis, therefore, are
Gdnf/Ret and
Pax2. In mice, deletion of
Gdnf and
c-Ret leads to renal agenesis or severe hypoplasia.
21,
60 Deletion of
Pax2, the “master organizer” of renal development, is incompatible with life.
60 The impact of genetic polymorphisms in these pivotal genes has been studied in humans. Haploinsufficiency of the
PAX2 gene causes the autosomal dominant renal coloboma syndrome, associated with significant reduction in nephron number and “oligomeganephronia.”
60,
62,
63 Taking this finding further, looking for a more subtle impact in the wider population, Quinlan et al. found that the common
AAA haplotype of
PAX2, present in 18.5% of newborns in a Canadian cohort, was associated with reduced allele-specific mRNA expression in vitro, and a 10% reduction in newborn kidney volume, compared with the
GGG haplotype.
63 Similarly, a polymorphic variant of
RET, RET(1476A), was also associated with reduced mRNA synthesis, an almost 10% reduction in kidney volume, and higher levels of the renal function marker cystatin C at birth compared with the
RET(1476G) variant in Caucasian newborns.
64 These authors found that newborn kidney volume is proportional to nephron number, therefore
PAX2 and
RET polymorphisms are likely associated with reduced nephron number in humans.
64 Among 15% of Caucasians inheriting both alleles, newborn kidney sizes were 23% smaller.
65 Surprisingly, however, none of 19 common
GDNF gene variants or three single nucleotide polymorphisms related to a putative
GDNF-PAX binding site were associated with small kidney size among 163 Caucasians newborns.
65 One rare coding GDNF variant (
R93W) was not found in any subject and therefore, the clinical impact of this potential mutation is not known.
65 These early and small studies suggest that genetic polymorphisms in genes that are critical in nephrogenesis may contribute to the wide spectrum of nephron number found in the general population.
Prematurity. Unlike in rodents, postnatal nephrogenesis does not occur in humans, except in extremely premature infants; therefore, nephron number is predominantly determined in utero. Rodriguez et al. examined kidneys from 56 extremely premature infants compared with 10 full-term infants at autopsy.
54 Radial glomerular counts were lower in premature compared with full-term infant kidneys and glomerular number correlated with gestational age, as has been reported previously.
54,
66 In addition, they found evidence of active glomerulogenesis (indicated by the presence of basophilic S-shaped bodies under the renal capsule in kidneys) in premature infants up to, but not beyond, 40 days of life.
54 This was the first study to demonstrate ongoing nephrogenesis in humans postnatally. Similarly, in preterm baboons, nephrogenesis was found to continue after birth and nephron number was within the normal range; however, there was a greater proportion of abnormal glomeruli in the superficial cortex compared to full-term controls, suggesting compromised nephrogenesis after premature birth.
67 In contrast, Hinchliffe et al. did not find an increase in nephron number in growth restricted infants who died as stillbirths at varying gestations, or at 1 year of age, suggesting a lack of nephrogenesis after birth.
66,
68 Gestational age was found to correlate with nephron number, which reached a maximum around 36 weeks.
69
Gender. Gender likely plays a complicated role in developmental programming. In the largest series of kidneys analyzed to date, glomerular number in adult females was found to be reduced by up to 12% compared to males.
13,
70 In a cohort of Cuban newborns, however, nephron number was not affected by gender.
48 In experimental models, reviewed in detail elsewhere, males generally tend to be more severely affected than females in terms of reduction in nephron number, as well as subsequent manifestation of hypertension and renal dysfunction.
71,
72 These differences may in part result from differences in postnatal growth rates between males and females, gender-specific differences in adaptation to adverse events, and gender-specific regulation of genes and pathways impacting renal development, function, and hypertension.
33,
72 Similarly, a large study in humans found an association of CKD with low birth weight in adult males, but not in females, suggesting a possible impact of gender on subsequent disease expression, although mechanisms are not yet clear.
73
Ethnicity. Hoy and colleagues have shown a reduction in nephron number among Aboriginal compared with non-Aboriginal Australians (
Table 2.1).
70 Among African Americans and Caucasian Americans, nephron number was not significantly different in both groups and correlated with birth weight, although the distribution appeared to be more bimodal in the African American cohort.
74 No low birth weight subjects were included in this study, but low birth weight is more prevalent among African Americans; therefore, in the general U.S. population, a greater proportion of African Americans may have lower nephron number. This remains to be studied. Nephron number among Senegalese Africans and African Americans was similar.
75 Among Cuban neonates, nephron number was again not different between black compared with white subjects.
48 To our knowledge kidneys of subjects from other ethnic groups have not been studied. Ethnicity, therefore, may have an impact on nephron number, although it is difficult to dissect out an impact independent of its association with birth
weight, socioeconomic factors, genetic polymorphisms, and many other potential confounders.
Intergenerational Factors. Among both white and African American women, mothers who had been of low birth weight had a significantly increased risk of having low birth weight offspring, independent of economic environment, suggesting a cross-generational effect of maternal low birth weight.
76 Similarly maternal, but not paternal birth weights, were associated with offspring birth weight, arguing for an intergenerational programming effect of the maternal environment.
77 Interestingly, in a large population-based study, mothers experiencing preeclampsia, especially when associated with premature birth and low birth weight in the offspring, are at increased risk of subsequent need for renal biopsy and/or ESRD.
47,
78 A reduced maternal glomerular filtration rate (GFR) <90 mL per minute and hypertension are significant risk factors for preeclampsia, small for gestational age infants, and premature delivery.
79 The question arises why the mother herself may have been predisposed to these adverse pregnancy-related and renal outcomes. It is conceivable that a vicious cycle may occur where a low birth weight mother would be predisposed to programmed adverse pregnancy outcomes, in turn impacting fetal nephrogenesis and thereby future pregnancy outcomes and renal health of the subsequent generations. To our knowledge this specific association has not been studied in humans. In rats, the first generation offspring of mothers fed low protein diets during gestation had low birth weights, low nephron number, and developed spontaneous hypertension at 8 weeks of age. Offspring of these first generation females, although maintained on normal diets throughout gestation, also exhibited low nephron number and hypertension, demonstrating intergenerational programming.
80 Interestingly the effect was lost by the third generation, suggesting that the intergenerational cycle can be interrupted by optimization of risk factors such as maternal nutrition.