Chapter 70A Cirrhosis and portal hypertension
Pathologic aspects
Overview
Cirrhosis is the final stage of a variety of chronic diseases involving the liver. Histopathologically, cirrhosis is characterized by remodeling of the vascular architecture with septal formation and nodular groups of regenerating hepatocytes. Biochemically, the cirrhotic liver functions less efficiently than the normal liver. Decompensated cirrhosis manifests as portal hypertension and liver failure, both of which are associated with numerous clinically important complications (see Chapter 70B, Chapter 71, Chapter 72, Chapter 73, Chapter 74, Chapter 75A, Chapter 75B, Chapter 75C, Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E, Chapter 77 ). In addition, regardless of the underlying etiology, cirrhosis is a major predisposing condition to the development of hepatocellular carcinoma (HCC) (Suriawinata & Xu, 2004). Portal hypertension secondary to cirrhosis develops as a result of the alterations of the hepatic microcirculatory system. Portal hypertension may also occur in the absence of the structural alterations of cirrhosis (noncirrhotic portal hypertension). The conditions associated with this may be idiopathic; all are subdivided into prehepatic, intrahepatic, or posthepatic. This chapter focuses on the pathology of cirrhosis and noncirrhotic portal hypertension and many of the conditions that result in portal hypertension.
Cirrhosis
The true prevalence of cirrhosis in the United States is unknown because many patients with compensated cirrhosis do not exhibit symptoms or signs of liver failure, but cirrhosis is among the top 10 causes of death in the Western world. In autopsy series, cirrhosis is documented in 5% to 10% of cases, but autopsy subjects may not be representative of the general population (Karsan et al, 2004). Currently, the only available and definitive treatment for established cirrhosis is liver transplantation.
Pathogenesis and Reversibility
The transformation from normal hepatic architecture to cirrhosis ultimately results from progressive deposition of fibrous tissue in regions of “extinct” parenchyma and vascular remodeling, regardless of the underlying etiology. Fibrogenesis is dynamic, and it is triggered by liver injury and mediated by a complex interplay of cellular necrosis, inflammation, apoptosis, and various cytokines. The cells that contribute to the development of hepatic fibrosis are the activated hepatic stellate cells and portal myofibroblasts. Once activated, these cell types are primed to respond to a wide variety of chemokines and cytokines (Friedman, 2008). In particular, transforming growth factor (TGF)-α seems to be a major fibrogenic mediator that induces these cells to produce collagens and other types of extracellular matrix and degradatory products (Canbay et al, 2004; Friedman, 2008; Pinzani & Marra, 2001; Ramadori & Saile, 2004). Recent work has shown the fibrogenic nature of leptin, the anorectic hormone produced in adipose tissue (LeClercq et al, 2002). During pathologic fibrogenesis, prevention of matrix degradation by metalloproteinases is orchestrated by the release of potent tissue metalloproteinase inhibitors; apoptosis of fibrogenic hepatic stellate cells also is inhibited. Scar formation ultimately is the consequence of the imbalance of collagen synthesis, deposition, and degradation. Another increasingly recognized pathway of fibrogenesis in the liver is that of epithelial-mesenchymal transformation (EMT), in which “injury” results in a cascade of cytokines that lead to epithelial cells developing and expressing mesenchymal markers (Rygiel et al, 2008); the exact role EMT plays in fibrogenesis is being studied.
Established cirrhosis has traditionally been considered irreversible. Accumulating clinical and experimental evidence suggests, however, that reversal or regression of liver fibrosis and cirrhosis may be possible (Fallowfield & Iredale, 2004). A study by Poynard and colleagues (2002) analyzed 3010 patients with chronic hepatitis C virus (HCV) included in four major clinical trials to receive randomized treatment regimens with interferon or pegylated interferon, with or without additional ribavirin. Reversal of cirrhosis was reported in 75 (49%) of 153 patients with baseline cirrhosis. Pol and associates (2004) examined 64 immunocompetent patients with HCV-related cirrhosis and found that cirrhosis disappeared in five patients (7.8%) on follow-up biopsies at a mean interval of 4.6 years. In addition, three of four patients undergoing dialysis showed reversal of cirrhosis secondary to HCV infection, and resolution of cirrhosis was shown in two patients upon examination of the whole liver explants at the time of transplantation.
More recently, Falize and colleagues (2006) analyzed 36 patients with hereditary hemochromatosis and demonstrated that regression of fibrosis was seen in 69% of those with bridging fibrosis and in 35% of patients with cirrhosis after institution of venosection therapy. Regression of cirrhosis has also been reported in patients with other diseases, such as hepatitis B virus (HBV), alcoholism, autoimmune hepatitis, and primary biliary cirrhosis (Arthur, 2002; Fallowfield & Iredale, 2004; Serpaggi, et al, 2006; Wanless et al, 2000). Spontaneous resolution of fibrosis also has been described in rats treated with carbon tetrachloride (Iredale et al, 1998; Issa et al, 2004). The proposed mechanisms for breakdown and remodeling of liver fibrosis include loss of activated stellate cells via apoptosis, decreased expression of metalloproteinase inhibitors, and increased production and activity of metalloproteinases or collagenases (Arthur, 2002; Benyon & Arthur, 2001; Fallowfield & Iredale, 2004; Gieling, et al, 2008). It is unclear, however, how these molecular and cellular events are initiated and regulated.
Currently, the extent to which cirrhosis is truly reversible is the subject of debate (Arthur, 2002; Chedid, 2000; Desmet & Roskams, 2003; Geller, 2000; Ray, 2000). Many important questions remain to be answered. Even if scar tissue is resorbed, can normal hepatic architecture be completely restored? Were there sampling differences or interpretation errors on biopsies in the studies showing reversibility? It is possibile that some of the reversed cases might have undergone a conversion of micronodular to macronodular cirrhosis, as shown in animal models (Issa et al, 2004), a condition that made the diagnosis of cirrhosis more difficult on a needle core biopsy sample (Desmet & Roskams, 2003)? Regardless, this discussion may have important clinical implications. Many liver diseases progress insidiously, and patients may seek medical attention only at the advanced stage, when treatment choices are limited. If cirrhosis is truly reversible, many patients with advanced liver disease still may benefit from medical treatment for specific etiologies, particularly with the discovery of new drug therapies.
Role of Liver Biopsy
In modern medicine, investigation of patients with chronic liver disease and cirrhosis involves multiple disciplines that include pathology, radiology, chemistry, biochemistry, virology, serology, and molecular biology. Liver biopsy evaluation remains the primary diagnostic tool, despite the drawback of invasiveness (Rockey et al, 2009). Noninvasive screening methods show promise in excluding patients with advanced fibrosis; however, in a recent study evaluating one of these tests, the positive predictive value for advanced fibrosis was still quite low, and the test fared poorly at distinguishing between intermediate stages of fibrosis (Zaman et al, 2007). For cirrhotic patients, liver biopsy can serve several important purposes, such as establishing or confirming diagnoses; assessing the possible underlying causes of disease; analyzing the grade of ongoing necroinflammatory activity; detecting dysplastic lesions or an occult HCC; and providing tissue for chemical, biochemical, molecular, or ultrastructural studies (Brunt, 2000a).
Two broad types of biopsy are used to sample liver tissue: needle biopsy and wedge biopsy. Needle biopsy has proved to be the most useful technique to obtain representative liver tissue for analysis. This procedure can be done percutaneously, transjugularly, or during open surgery or laparoscopy; a cutting needle or the Menghini aspiration needle may be used, although the former generates a better biopsy specimen. If cirrhosis is suspected clinically, a cutting needle is the preferred method of biopsy because an aspiration needle often provides a fragmented specimen that makes histologic evaluation difficult. The size of the needle biopsy specimen is important in avoiding sampling error. Traditionally, it has been recommended that an adequate biopsy specimen should be no smaller than 20 gauge and at least 1.5 cm in length, or it should contain at least five portal tracts (Afdhal & Nunes, 2004; Rockey et al, 2009). It has been suggested more recently, however, that for accurate and reliable grading and staging of chronic viral hepatitis, a biopsy specimen of 2 cm in length or longer that contains at least 11 complete portal tracts is needed (Guido & Rugge, 2004).
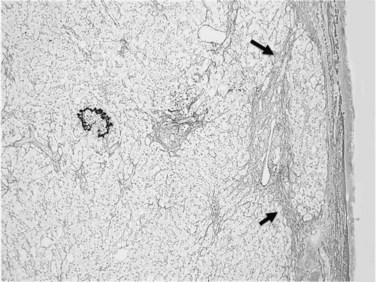
Usually performed during open surgery or laparoscopy, wedge biopsy is discouraged for evaluation of diffuse parenchymal liver diseases because this technique samples primarily the subcapsular liver parenchyma, which may contain misleading fibrous septa extending from the capsule that could be easily confused with cirrhosis or bridging fibrosis (Fig. 70A.1). The same issue can also occur in a subcapsular needle biopsy. In addition, nonspecific necroinflammatory change can occur quickly in the subcapsular region during surgical procedures, which may further confuse histology. A wedge biopsy is more suitable for focal lesions present on or immediately below the capsule. Even during open surgery, a needle biopsy to sample deep liver parenchyma is preferred (Guido & Rugge, 2004).
Morphology
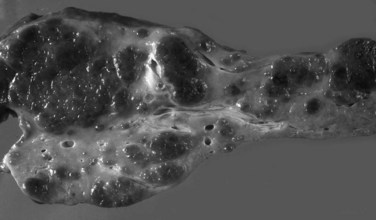
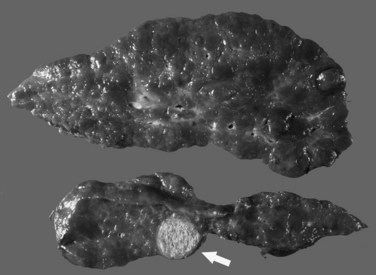
Grossly, the liver with established cirrhosis exhibits a nodular appearance that diffusely involves the entire liver (Fig. 70A.2). Microscopically, the liver parenchyma is divided by variable-sized fibrovascular septa, which contain profiles of ductular structures and diverse inflammatory cell types, into nodules that typically no longer retain a terminal hepatic venule or identifiable portal tracts. The normal portal-central relationship is completely effaced; the exception to this is biliary cirrhosis, in which the terminal hepatic venule may retain its central location. The hepatocytes within the nodules may appear morphologically normal, or they may show evidence of regeneration. The latter may be characterized by thickened cell plates with up to two cells across; anisonucleosis; large cell change with maintenance of nuclear/cytoplasmic (N/C) ratio; or small, crowded cells with markedly increased N/C ratios. The reticulin stain is useful to show cord thickening. The increased ductular profiles (ductular proliferation) are referred to as a ductular reaction (Roskams et al, 2004). Some cases may retain lymphoid aggregates in the septa, and some may have interface hepatitis.
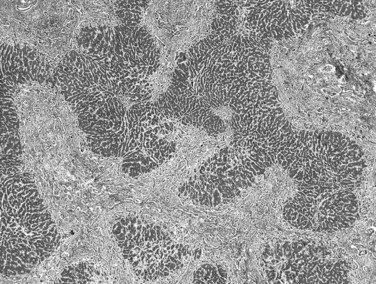
Biliary cirrhosis is caused by disorders such as primary biliary cirrhosis, primary or secondary sclerosing cholangitis, and biliary atresia. This type of cirrhosis exhibits unique morphologic features characterized by a highly irregular “jigsaw puzzle” or “geographic” nodular pattern (Fig. 70A.3). As noted, the terminal hepatic venule may be retained, and loss or effacement of native bile ducts by either lymphoid aggregates or scar tissue may be evident. Ductular reaction (proliferation) may be more pronounced than that seen in other types of cirrhosis. A characteristic clue to biliary cirrhosis is the constellation of findings in periseptal hepatocytes referred to as cholate stasis. These findings include periseptal hepatocyte ballooning, Mallory-Denk bodies (Mallory’s hyaline), and copper deposition. In addition, foamy cell aggregates may be seen in the nodules. Some investigators have attributed large cell change to chronic cholestasis. Another subtle histologic clue to biliary cirrhosis is the presence of nodular regenerative hyperplasia in the regenerative nodules.
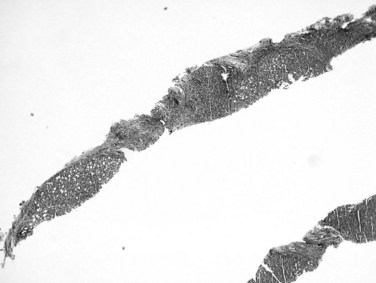
Recognition of cirrhosis is usually straightforward when an adequate biopsy specimen is examined, even on hematoxylin and eosin–stained sections. It may be helpful to use Masson trichrome stain to highlight the dense perisinusoidal fibrosis of alcoholic hepatitis and cirrhosis, particularly for fragmented needle biopsy specimens (Fig. 70A.4). Fragmentation of the specimen noted at the time of biopsy should raise the suspicion of cirrhosis. On histologic sections, a thin layer of collagen, better appreciated on trichrome and reticulin stains, tends to adhere to the surface of detached nodules. Cirrhosis also may be difficult to diagnose on needle biopsy specimens, when a macronodule is sampled, or when cirrhosis is incomplete (incomplete septal cirrhosis). In the last case, the complete spectrum of morphologic features of cirrhosis are not exhibited but vascular relationships are markedly altered, and ectatic, eccentrically located portal veins may be noted. In addition, the septa are thin, and some may be seen to extend into the parenchyma and end blindly. This type of “cirrhosis” is not uncommon in HBV-related liver disease.
Cancer Risk
The most common cancer that arises in cirrhotic livers is HCC (Fig. 70A.5; see Chapter 80), although cholangiocarcinoma is being increasingly recognized in cirrhosis (see Chapter 50A; Shaib, et al, 2007). Although these cancers may occur in cirrhosis of any etiology, particular risks include HBV and HCV infection, alcoholic liver disease (ALD), and hemochromatosis. Grossly, HCC may be multifocal, or it may grow as a well-circumscribed mass; it may show evidence of necrosis, or it may possess a different color and texture from background cirrhotic nodules. It also may bulge from the cut surface on sectioning. Most cases of HCC arising in cirrhotic livers are found in nodules larger than 1 cm (Bruix et al, 2001). Studies have shown, however, that nodules smaller than 1 cm can be malignant in the setting of cirrhosis (Caturelli et al, 2004). Premalignant lesions, or dysplastic nodules, may be indistinguishable from HCC by gross examination.
Cholangiocarcinoma (CCa) in cirrhosis may or may not be distinguishable grossly from HCC. The diagnosis rests with the microscopic appearance of atypical glandular structures, commonly in desmoplastic stroma. CCa also develops in longstanding primary sclerosing cholangitis and is seen more often than HCC in noncirrhotic livers (see Chapter 50A). The tumor may infiltrate the liver diffusely, or it may occur as a mass lesion that may be intrahepatic or extrahepatic. The cirrhotic liver is considered to be resistant to metastases from extrahepatic tumors.
Not all large nodules detected in cirrhotic livers are HCC. A macroregenerative nodule or large regenerative nodule usually measures 0.5 to 1.5 cm and is rarely 5 cm or more in diameter (International Working Party, 1995). It is seen more commonly in macronodular cirrhosis and may be distinct from surrounding cirrhotic nodules on gross examination. Histologically, a macroregenerative nodule may contain portal structures or short fibrovascular septa. The hepatocytes within the nodule are similar to the hepatocytes in smaller cirrhotic nodules but almost always exhibit hyperplastic change, evidenced by thickened plates. The clonal nature of the macroregenerative nodule has been shown, and its malignant potential is an area of ongoing discussion (Bailey & Brunt, 2002).
A dysplastic nodule is a premalignant lesion that usually measures more than 0.5 cm (International Working Party, 1995). Evolution to HCC within months or a few years has been well documented (Hytiroglou, 2004). A high-grade dysplastic nodule exhibits more clear-cut architectural or cytologic atypia, such as bulging or maplike clonal growth; pseudoglandular formation; unpaired arteries; and small cell change characterized by increased cell density, high nuclear/cytoplasmic cell ratio, and nuclear hyperchromasia. These morphologic changes are insufficient, however, for the diagnosis of HCC because a dysplastic nodule does not invade the surrounding stroma or blood vessels and it maintains cell plates no more than three cells wide (Roncalli, 2004).
Distinguishing a high-grade dysplastic nodule from well-differentiated HCC can be extremely difficult or impossible from a needle biopsy specimen, although it may be less ambiguous when an explant is examined (Kojiro, 2004). Identification of ductular reaction at the periphery aids in positive identification of cirrhosis or dysplastic nodule (Park et al, 2007; Lennerz et al, 2009). Clinical management of patients with cirrhosis and dysplasia in biopsy specimens is challenging.
Assessment of Underlying Etiology
Cirrhosis is best classified by its underlying etiology (Box 70A.1), which can be determined by clinical history and laboratory investigation in many but not all cases. Morphologic examination may help establish the diagnosis or guide the clinical investigation. In the following discussion, the morphologic features characteristic of many chronic liver diseases that commonly cause cirrhosis are illustrated. At end-stage liver disease, however, the histopathologic findings may be nonspecific and indiscriminate even to experienced hepatopathologists. Many cases of “cryptogenic” cirrhosis represent burned-out processes, for which no identifying clinical or morphologic features remain. These cases are frequently due to ALD, autoimmune hepatitis, and nonalcoholic fatty liver disease (NAFLD). The contribution of as yet unnamed viral agents is not known but is recognized.
Alcoholic Liver Disease
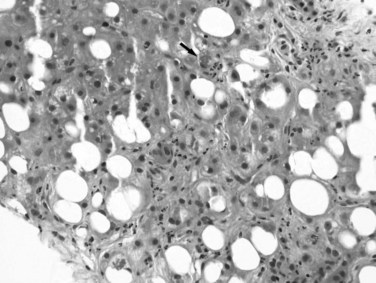
Excessive alcohol consumption is the leading cause of liver disease in the Western world, which encompasses a clinicopathologic spectrum that includes fatty liver, alcoholic hepatitis, and alcoholic cirrhosis (Haber et al, 2003; Menon et al, 2001; O’Connor & Schottenfeld, 1998; Tome & Lucey, 2004). Alcoholic cirrhosis, or Laënnec cirrhosis, is classically micronodular and may retain some of the features of alcoholic hepatitis at the initial stage. The liver may appear pale or yellow, enlarged, and greasy on gross examination. Histologically, lesions predominate in the perivenular region (zone 3) of the acinus and include various combinations of steatosis (fatty change), ballooning, Mallory-Denk bodies, and satellitosis. Steatosis may be predominantly macrovesicular, defined by the presence of large fat droplets in the cytoplasm of hepatocytes displacing the nuclei. Microvesicular steatosis also can be seen, characterized by fine fat droplets surrounding the central nuclei. Alcoholic hepatitis is characterized by an inflammatory infiltrate rich in neutrophils, most frequently distributed in the lobules adjacent to hepatocytes containing Mallory-Denk bodies, and dense, perisinusoidal fibrosis (Fig. 70A.6). Lymphocytes and histiocytes also may be present, sometimes in the form of lipogranulomas, and megamitochondria also may be evident. In the portal and periportal regions, ductular reaction with numerous neutrophils may occur. Cholangiolitis and canalicular bile plugs are worrisome lesions for concomitant pancreatitis.
The patterns of fibrosis in alcoholic hepatitis are characteristic. Fibrosis usually involves the terminal hepatic venules, leading to the thickening of the wall; luminal occlusion may be seen with necrosis of adjacent hepatocytes and Mallory-Denk bodies within remaining hepatocytes, a lesion referred to as central hyaline necrosis. Subendothelial fibrosis is a venoocclusive lesion of alcoholism that may be noted even in end-stage cirrhotic livers. Fibrosis may extend into the lobules in perisinusoidal spaces as delicate or dense strands, giving rise to a distinctive “chicken wire” pericellular (perisinusoidal) distribution (Brunt, 2002). Trichrome stain is particularly useful in detecting this unique form of fibrosis. With time, the liver may be replaced by micronodular cirrhosis; often, the septa of ALD are quite broad as manifestations of the microvascular obliteration.
Nonalcoholic Fatty Liver Disease
NAFLD is increasingly recognized as a significant form of potentially progressive liver disease. Prevalence studies estimate that approximately 20% to 25% of the U.S. population is affected by fatty liver; of these individuals, 15% to 20% are at risk for progression to cirrhosis (Angulo, 2002). The lesions noted in liver tissue may resemble many of the features of alcohol-induced liver damage in individuals who are not heavy drinkers (Brunt, 2004; Salt, 2004; te Sligte et al, 2004; Zafrani, 2004). The disease is etiologically attributed primarily to insulin resistance and is considered the hepatic manifestation of the metabolic syndrome, a constellation of obesity, hypertension, diabetes, and hyperlipidemia (Marchesini et al, 2001). Studies have also correlated the lesions of nonalcoholic steatohepatitis (NASH) with features of metabolic syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree