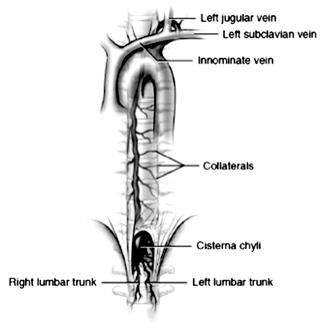
The thoracic duct is formed from downward growth of the jugular sacs and upward growth of the cisterna chyli [11]. In the embryo, the thoracic duct exists as bilateral symmetrical plexus of lymphatic vessels. The communicating vessels enlarge and fuse, eventually leading to obliteration of the upper third of the right duct and the lower two-third of the left duct leaving the adult thoracic duct. The plexus of lymphatic drainage results in multiple connections between the thoracic duct and adjacent veins, including the azygos and intercostals, and allows chyle to reach the blood stream after duct ligation.
Anatomy
The anatomy of the thoracic duct is known for its variability. The cisterna chyli originates in the abdomen from the union of two lumbar lymphatic and one intestinal trunk (Fig. 5.2 ). The standard position of the cisterna chyli is adjacent to the vertebral column and to the right of the aorta at the level of L2, but it can be found from T10 to L3. The thoracic duct ascends from the cisterna chyli in the posterior mediastinum through the aortic hiatus. The aortic hiatus resides at the level of T10. Moving cephalad, the duct lies along the anterior surface of the vertebral column, posterior to the esophagus, between the aorta and the azygos vein and anterior to the right intercostal arteries. This anatomic region is emphasized because this is the optimal location for duct ligation in the chest [12]. The thoracic duct typically crosses behind the aorta to the left at T5–T7 and continues its ascent behind the aortic arch and to the left of the esophagus until it reaches the level of the left subclavian artery posteriorly. The change in laterality of the duct position explains the development of a right-sided chylothorax if the duct is injured below T5 and a left-sided chylothorax if the duct is injured above T5. Collateral drainage into the azygos, intercostal, and lumbar veins occurs 40–60 % of the time.
The course of the duct continues cephalad until approximately 3 cm above the level of the clavicle when it traverses laterally. The duct is then positioned anterior to the vertebral artery and vein, innominate vein, and phrenic nerve and medial to the anterior scalene muscle. The duct terminates by joining the venous drainage system near the confluence of the left subclavian and left internal jugular veins, but has also been reported to drain into the left innominate vein, left or right internal jugular or the left vertebral vein (Fig. 5.3 ) [13–15].
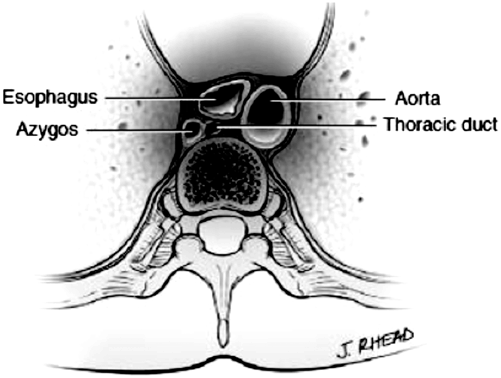

Fig. 5.3
Adult anatomy of the thoracic duct, relationship to mediastinal structures. (Reprinted with permission from [48])
The duct is known to have unidirectional valves of variable number and location. A valve is always present, however, at the junction of the thoracic duct and the venous supply to protect against the reflux of blood into the lymphatic system [16].
Other small lymphatic pathways exist. A small and short right thoracic duct drains lymph from the right head, neck, arm and chest wall via the jugular trunk. A bronchomediastinal trunk drains lymph from the right lung, heart, and left lung. And an additional trunk drains lymph from the dome of the liver, right chest wall, and right diaphragm via the right internal mammary trunk.
Variations in anatomy include lymphatic duct doubling, left-sided course, right-sided course, bilateral termination, or azygos vein termination. In addition, in the neck, the duct can run posteriorly to the vertebral and subclavian veins [17].
Physiology
Chyle consists of lymph (comparable to blood plasma) and emulsified fats (free fatty acids). It is formed within the small intestine during digestion of fatty foods. Long-chain fatty acid molecules diffuse into the low-pressure wall of the intestinal villi. They form micelles and are reassembled into triglycerides. The triglycerides are coated with cholesterol and protein to form chylomicrons that then enter lacteals before flowing into the larger lymphatic vessels. The higher pressure in intestinal veins allows only smaller products of digestion, such as short- and medium-chain triglycerides (MCT), amino acids and sugars, to diffuse directly into the blood stream, the portal system. Fat is absorbed into intestinal lymphatics and transported into venous blood flow in less than an hour.
Lymph flow in the thoracic duct comes from the liver, intestines, and extremities, with the liver and intestines contributing 95 %. Many factors influence the volume of lymph flow through the thoracic duct. Basal rate of flow is estimated to be 0.95 ml/min or 1.38 ml/kg body weight/hour. Flow rates can increase with oral intake and abdominal massage up to 3.9 ml/min [18].
Composition of Chyle
The concentration of fat, protein, and lymphocytes within chyle is variable depending on the timing, type, and amount of food ingested. During the period of fasting, ductal lymph fluid is clear. The milky white color occurs from the absorption of chylomicrons following fat ingestion. Chyle is considered bacteriostatic and causes a very little pleural reaction due to its alkaline pH (Table 5.1).
Component | Amount (per 100 ml) |
|---|---|
Total fat | 0.4–5.0 g |
Cholesterol | 65–220 mg |
Protein | 2.2–5.9 g |
Albumin | 1.2 − 4.1 g |
Globulin | 1.1–3.6 g |
Fibrogen | 16–24 g |
Antithrombin | 25 % of plasma concentration |
Prothrombin | 25 % of plasma concentration |
Fibrinogen | 25 % of plasma concentration |
Sugar | 48–200 g |
Electrolytes | Similar to plasma |
Cellular elements | |
Lymphocytes | 400 − 6800/L |
Erythrocytes | 50–600/L |
Lipids
As noted, the main component of chyle consists of emulsified fats, or free fatty acids. The concentration of fat in chyle is directly related to ingested quantity and composition of fat and can range from 14 to 210 mmol/L. Up to 60 % of ingested fat, consisting mostly of long-chain triglycerides (12 or more carbon atoms in size), is absorbed into the lymphatic channels. Small-chain triglycerides, considered less than 6 carbon atoms in size, are absorbed directly into the portal venous system. MCT (6–12 carbon atoms in size) are also absorbed passively into the portal system, though only 30–40 % of MCTs are directly absorbed.
Protein
Chyle is a transporter of extravascular protein back to the vascular space. Total protein concentration in chyle is generally half that of protein concentration in the plasma, ranging from 21 to 59 g/L [18]. In large chyle leaks, significant protein losses can occur.
Electrolytes
The electrolyte content of lymph in the thoracic duct is the same as that of plasma. Fat-soluble vitamin concentrations in chyle are proportional to the amount ingested. Pancreatic lipase, amylase, and deoxyribonuclease can also flow into the lymph system and are subsequently transported to the blood stream by way of the thoracic duct.
Lymphocytes
Lymphocytes contribute the main cellular element of thoracic duct lymph. Ninety percent are T-lymphocytes. Lymphocytes are in constant to and fro circulation from lymph nodes to the bloodstream. Prolonged drainage of lymph due to a thoracic duct injury can significantly deplete lymphocytes with resultant immunosuppression.
Chylothorax
Etiology/Cause
Chylothorax occurs when lymphatic fluid accumulates within the pleural space. Though a chylothorax can occur spontaneously, it is usually related to an injury to the thoracic duct or one of its branches. Other causes include occlusion of the lymphatic system from venous thrombosis, neoplastic infiltration, or radiation. The causes are listed in Table 5.2.
Congenital anomalies |
|---|
Trauma |
Birth trauma |
Blunt trauma |
Penetrating trauma |
Surgical trauma |
Cervical lymph node dissection |
Thoracic |
Ligation of patent ductus arteriosus |
Coarctation repair |
Esophagectomy |
Thoracic aortic aneurysm repair |
Resection of mediastinal tumor |
Pulmonary resection |
Sympathectomy |
Abdominal |
Abdominal lymph node dissection |
Neoplasms |
Lymphoma, breast cancer, lung cancer |
Miscellaneous |
Subclavian vein thrombosis |
Radiation |
Tuberculosis |
Post-esophagectomy Chylothorax
Thoracic operations most commonly associated with chylothorax include aortic procedures (incidence of 0.2–0.5 %), pulmonary resection with lymphadenectomy (incidence of 0.42–2.3 %.), and esophagectomy. The incidence of chylothorax after esophagectomy ranges from 0.5 to 10.5 %, irrespective of the approach to resection [19–22]. A meta-analysis completed by Rindani and colleagues evaluated 44 reports involving 5483 patients with an incidence of chylothorax of 2.8 % [23]. Patients who had a transthoracic esophagectomy (2675) and those who had a transhiatal esophagectomy (2808) developed chylothoraces with an incidence of 2.1 and 3.4 %, respectively. In a report by Dugue of 850 patients undergoing Ivor-Lewis esophagectomy, the incidence of chylothorax was 2.7 % [24]. Orringer reported < 1 % incidence for 1085 patients who underwent a transhiatal esophagectomy [25]. Merigliano reported 1787 esophagectomies with an incidence of chylothorax of 1.1 % [26]. Of the 1787 patients evaluated, 1237 patients underwent a transthoracic approach and 464 patients had a transhiatal approach with chylothorax incidence of 1 and 1.3 %, respectively. Minimally invasive esophagectomy (MIE) has reported rates of chylothorax similar to those of open approaches. Shen reported 344 MIEs with a chylothorax incidence of 2.9 % [27]. A postoperative chyle leak is also more likely to occur in direct relationship with the aggressiveness of a mediastinal lymph node dissection [28].
Diagnosis
Clinical Features
Clinical features related to chylothorax often present in a delayed fashion because postoperative patients frequently have a limited dietary intake. As oral or enteral intake occurs, lipids are absorbed through the intestinal tract and into the lymphatic system that travels through the region of the resected esophagus. If thoracic duct channels have been disrupted and are not ligated, the pleural cavity will gradually fill with chyle. Clinical complaints are related to compression of the lung by the chylous effusion and include dyspnea, cough, and fatigue. If pleural drainage tubes are present, a milky effluent will occur. The quantity of accumulated or drained fluid depends upon the degree of thoracic duct injury and amount of enteral intake. High-volume drainage (> 1–2 L/day) can occur with losses of fluid, electrolytes, and lymphocyte reserves.
Fluid Studies
After thoracentesis or catheter drainage of the suspected effusion, the diagnosis of chylothorax is supported by nonclotting, milky-colored fluid. Chyle can resemble pus, but it is odorless, and no bacteria are seen on Gram stain. Clear fluid does not rule out chylothorax, particularly in patients on limited diets. The rate of daily fluid accumulation, alone, is a key piece of data. A higher-than-usual volume of serous drainage (700–1200 ml/day) is characteristic of a thoracic duct injury and chylothorax. In such circumstances, a complete blood count of the fluid with differential that shows lymphocytes > 90 % is diagnostic.
Biochemical and microscopic examination of the pleural fluid is important. Diagnostic findings include triglyceride level > 110 mg/dL and/or a concentration greater than plasma triglyceride level. Pleural fluid triglyceride concentrations, however, can be less than 110 mg/dl in 15 % of patients with a chylothorax. Therefore, lipoprotein analysis can be performed as another diagnostic tool. A microscopic examination that shows chylomicrons is also diagnostic of a chylothorax and can be used as a confirmatory test if the triglyceride levels are equivocal. On microscopy, fat globules will clear with alkali or ether and will stain with Sudan III.
Imaging
Chest radiography and computed tomography will often show a unilateral pleural effusion in an undrained chest cavity. Other findings can include bilateral effusions, a widened mediastinum, and a pericardial effusion. Though uncommonly performed and usually unnecessary, lymphangiography can show the site of injury [29]. This procedure involves injection of 10 mL of ethiodized oil into the lymphatic vessels in the dorsum of the foot. Coupled with lymphangiography, post-procedure computed tomography of the chest can be highly accurate in localizing a chyle leak [30].
Treatment
The best treatment of chylothorax is prevention. Attention to the anatomy of the thoracic duct and its variability is required to avoid injury to the structure and its tributaries. Because of the proximity of the thoracic duct to the esophagus and aorta, intrathoracic aortic and esophageal procedures carry a particular risk for duct injury. The judicious use of tying and clipping of the lymphatic, periaortic, and periesophageal tissues during dissection minimizes the risk of chylothorax occurrence. The duct and lymphatic channels are not often visualized at the time of surgery because flow through the duct system is minimal as a result of a patient’s nil per os (NPO) status prior to surgery. If the duct must be visualized during an operation, for inspection or repair, 30 cc’s of fluid that is rich in fat (milk or olive oil) can be given orally or through a nasogastric tube 1 h prior to anticipated exposure of the duct. Another method to prevent postoperative chyle leakage is ligation of the thoracic duct at the level of the aortic hiatus. Guo and colleagues reported a group of 135 minimally invasive esophagectomies for cancer [31]. Of the 65 patients who had prophylactic thoracic duct ligation, one patient developed a chylothorax, whereas 7 chylothoraces occurred in 65 patients who did not have ligation of their ducts. No complications occurred from duct ligation.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree