Chapter 43 Cholangitis
Cholangitis
Cholangitis is not a single disease with a well-defined clinical appearance but rather a spectrum of disease that presents variably with a wide range of severity. The appearance and course of cholangitis ranges from mild, intermittent, and recurrent episodes of abdominal pain, jaundice, fever, and chills as described by Charcot in 1877 to a rapidly progressive systemic illness that results in shock, coma, and death as described by Reynolds and Dargan (1959).
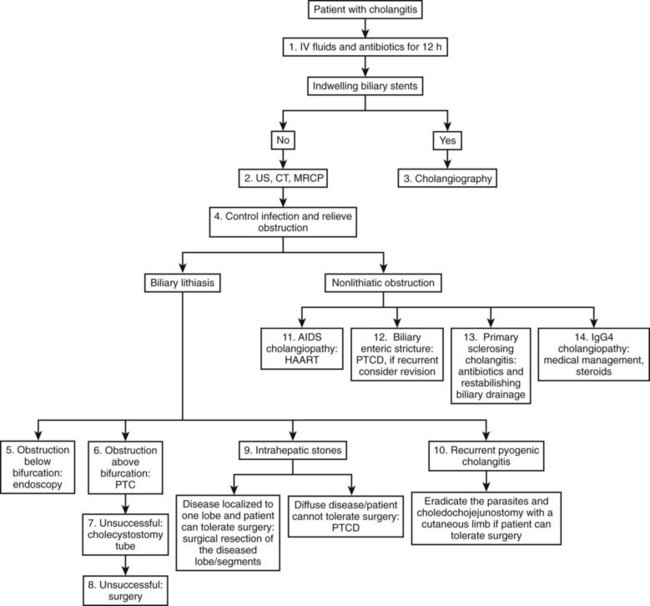
The development of cholangitis requires the presence of three factors: 1) obstruction of bile flow, 2) colonization of the bile with bacteria (bactobilia) or fungi, and 3) elevation of intraductal biliary pressure. The most common causes of biliary obstruction associated with cholangitis are calculi, benign and malignant strictures, obstructed stents, and parasites (Table 43.1; see Chapters 7 and 11). The management of cholangitis should follow three principles: 1) vigorous resuscitation and hemodynamic support, 2) broad-spectrum parenteral antibiotics, and 3) relief of biliary obstruction (decompression). Figure 43.1 provides a suggested management algorithm for patients with cholangitis.
Table 43.1 Causes of Cholangitis
AIDS, acquired immunodeficiency syndrome; ERCP, endoscopic retrograde cholangiopancreatography; PTC, percutaneous transhepatic cholangiography
Modified from Bornman PC, et al, 2003: Management of cholangitis. Hepatobiliary Pancreat Surg 10:406-414.
Pharmacologic Treatment for Cholangitis
The pharmacologic treatment of cholangitis differs from that of acute cholecystitis in the need for more specific and broader antimicrobial therapy dictated by the patient’s underlying pathology and clinical condition. Culture-identified bacteriology of the biliary tree has changed over the past 40 years. Previously, the gram-negative aerobes Escherichia coli and Klebsiella, gram-positive aerobes, and enterococci were the most common isolates identified from patients with cholangitis (Helton, 1987). More recently, infections have been polymicrobial in 30% to 80% of cases (Westphal & Brogard, 1999). In some studies, anaerobes have been detected in more than 15% of patients but rarely as the sole isolate. Bacteroides and Clostridium species are the most frequently cultured anaerobes, and anaerobic bacteria are commonly isolated from biliary tract specimens from patients who have a history of biliary surgery, especially those with a biliary-enteric anastomosis or chronic biliary tract infection and the elderly. Cholangitis arising from anaerobic organisms is reported to be associated with a more severe clinical illness compared with purely aerobic infections (Csendes et al, 1996; see Chapter 11).
Bacteremia in acute cholangitis has been demonstrated to be the result of increased bile duct pressure that favors the reflux of bacteria into the blood and lymphatic circulation (Csendes et al, 1995); it has been reported in 21% to 71% of patients with cholangitis (Cotton et al, 1991; Csendes et al, 1996; see Chapter 7). The organisms isolated reflect a similar distribution to that of biliary cultures, except for anaerobes and enterococci, which are infrequently found in blood cultures.
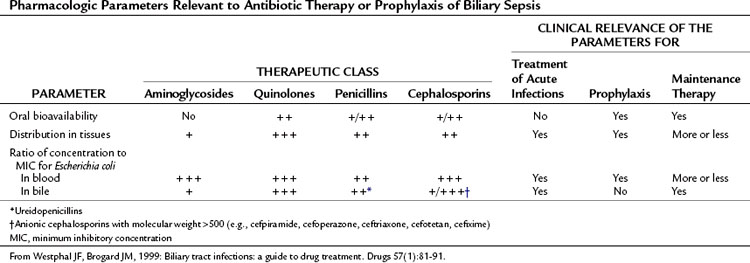
A variety of antibiotic regimens and combinations have been compared in prospective randomized clinical trials to establish efficacy, safety, and toxicity profiles. Monotherapy with broad-spectrum agents, such as a third- and fourth-generation cephalosporins (cefotaxime, cefipime), and a ureidopenicillin (mezlocillin, piperacillin) combined with a β-lactamase inhibitor (ticarcillin-clavulanate, piperacillin-tazobactam) or quinolone (ciprofloxacin) have been reported to be as effective in treating patients with cholangitis as metronidazole or clindamycin in combination with an aminoglycoside or a third-generation cephalosporin and ampicillin (Sung et al, 1995; Thompson et al, 1993).
In a multicenter comparative study of cefepime versus broad-spectrum antibacterial therapy in moderate and severe bacterial infections (Badaro et al, 2002), cefepime was demonstrated to achieve a higher cure rate compared with broad-spectrum combination therapy as an initial empiric treatment for hospitalized patients with moderate to severe community-acquired infections.
Carbapenems, ureidopenicillins, and fluoroquinolones offer good coverage for gram-negative aerobes (Mazuski et al, 2002), but piperacillin offers the advantage of gram-positive coverage, including enterococci, as well as anaerobic coverage (Thompson et al, 1990). Tazobactam, a β-lactamase inhibitor, extends the spectrum to cover organisms that have acquired resistance. These regimens are sufficient for most patients presenting with de novo cholangitis who have not yet been hospitalized, operated upon, or instrumented (Table 43.2). Again, it is emphasized that although hydration and antibiotics may improve the clinical condition in up to 80% of patients with acute cholangitis, 20% with clinical sepsis will require urgent biliary decompression.
For patients with a previously instrumented biliary tract (endoscopic retrograde cholangiopancreatography [ERCP], placement of a biliary stent), a previous biliary operation, or prolonged hospitalization, the cholangitis is likely to be associated with resistant flora such as Pseudomonas or Serratia species. Liver transplant patients who develop cholangitis have been observed to have Candida and/or Enterococcus in the biliary tree. Interestingly, vancomycin-resistant enterococci (VRE) are common in liver transplant patients (Schlitt et al, 1999). When Candida and/or Enterococcus are cultured, these should be treated with amphotericin and one of the streptogramins (quinupristin/dalfopristin [Synercid] or oxazolidinone linezolid [Zyvox], respectively). For all hospitalized patients, antibiotic coverage should always take into consideration the local resistance patterns (“antibiotic-gram”) of specific pathogens.
Duration of Antibiotic Therapy for Cholangitis
No randomized clinical trials have firmly established the duration of antibiotic therapy for the treatment of cholangitis. However, general guidelines are for antibiotic therapy to continue until biliary obstruction is completely relieved, biochemical liver function tests have improved or normalized, and the patient is afebrile for at least 48 hours. After successful biliary drainage by ERCP, a retrospective study comparing short-duration antibiotic therapy (3 days) with longer antibiotic coverage showed that 3 days of antibiotic therapy appears sufficient when adequate drainage has been achieved and fever is abating (van Lent et al, 2002).
Diagnostic Imaging Studies
Transabdominal Ultrasound
TUS (see Chapter 13) is the least expensive and most universally available imaging modality to evaluate the biliary tree and gallbladder. As such, it is the preferred first diagnostic test when evaluating a patient with acute cholecystitis and/or cholangitis. US can reliably demonstrate intrahepatic and extrahepatic biliary dilation, but this can be absent in the case of an acute obstruction. In the presence of gallstones less than 10 mm in size and dilation of the CBD greater than 10 mm, CBD obstruction by gallstones is likely (Abboud et al, 1996). The sensitivity of US to detect CBD stones varies between 20% and 75%, with increased sensitivity in the case of multiple large stones within a dilatated CBD and less sensitivity in the case of stone impaction in the retropancreatic segment of the CBD.
Computed Tomography
Non–contrast-enhanced CT scan (see Chapter 16) overcomes the technical limitations of US, as it can detect impacted stones in the retropancreatic CBD segment; when performed with 2- to 3-mm slices, its sensitivity for detecting small stones is reported to range between 65% and 80% (Neitlich et al, 1997). However, because up to 25% of gallstones will have the same density as bile, the sensitivity of noncontrast CT does not exceed 80%.
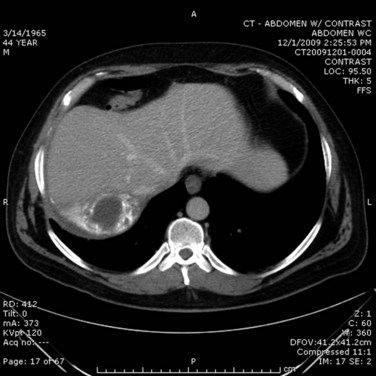
In the case of cholangitis, intravenous (IV) contrast–enhanced CT scan can reveal inflammation and demonstrate thickness of the bile ducts. The arterial phase should reveal peripheral or periductal hypervascularization (Arai et al, 2003). Another important role for CT is to detect the complications of cholangitis, such as hepatic abscess (Fig. 43.2) and pylephlebitis (suppurative thrombosis of the portal vein). Finally, CT can reveal etiologies of biliary obstruction, other than a CBD stone, such as a tumoral mass; however, contrast material is associated with a risk of hypersensitivity or induced renal toxicity, particularly in underresuscitated patients or in the presence of hemodynamic instability.
Magnetic Resonance Cholangiopancreatography
Magnetic resonance cholangiopancreatography (MRCP; see Chapter 17) provides imaging in a noninvasive manner that allows visualization by emphasizing the hyperintensity of stationary liquids, thus the use of this modality precludes the need for contrast injection to fully visualize the biliary system. Images obtained by MRCP are similar to those obtained by cholangiogram: stones appear as hypointense defects (lacking liquid) within the biliary system. However, MRCP cannot differentiate stones from air bubbles, sludge, or blood clots—all these lack liquid. A further limitation of MRCP is that it cannot detect stones less than 3 mm in size, nor can it detect impacted stones in the ampulla, which require evaluation of T1 images before and after gadolinium injection. The overall sensitivity of MRCP to detect CBD stones is 80% to 100%, and its specificity is excellent, varying between 90% and 100% (Hakansson et al, 2002; Kondo et al, 2001; Soto et al, 2000). It is important to recall that MRCP is purely a diagnostic modality, it is not therapeutic.
Endoscopic Ultrasound
Endoscopic ultrasound (EUS; see Chapter 14) is an invasive procedure that requires sedation or general anesthesia. Once it has been passed through the stomach and into the duodenum, the US probe is in close relationship to the CBD; by using a high US frequency (7.5 to 12 MHz), the resolution of EUS is exceptional (<1 mm), making it the gold standard in the detection of small gallstones or other obstructive etiologies. The major limitations of EUS are that it cannot be performed in patients with altered anatomy, such as those with a prior distal gastrectomy or gastric bypass, or in the presence of a significantly calcified pancreas or hilar biliary pathology.
Direct Cholangiography
Although historically considered the reference radiologic modality, direct cholangiography (see Chapter 18) for purely diagnostic purposes has been supplanted by less invasive cross-sectional imaging alternatives, which also provide additional anatomic detail related to adjacent organs to allow a more complete assessment. Retrograde or percutaneous cholangiography is largely limited to the first step of a therapeutic procedure (Gallix et al, 2006).
In addition to its inferior image quality, there are various complications associated with the use of direct cholangiography, including biliary infection, pancreatitis, and hemorrhage (Vidal et al, 2004).
Management of Cholangitis
Extrahepatic Biliary Stone Obstruction: Procedures for Biliary Decompression
When performed in a timely manner, biliary decompression and successful drainage are the cornerstones of acute cholangitis treatment. Several techniques can be used to ensure effective biliary drainage. A review of these techniques with the Tokyo guidelines was recently published (Tsuyuguchi et al, 2007).
The preferred method and route to relieve biliary obstruction, and the ultimate success of biliary drainage, depends upon a number of factors that include availability of the technique (endoscopic, percutaneous, or operative), local expertise, etiology, location and severity of obstruction, and the presence of comorbid medical conditions. Patients who do not respond to initial medical treatment within 12 to 24 hours should undergo emergent, nonoperative decompression of the biliary tract. When the obstruction is believed to be below the bile duct bifurcation, the preferred initial approach is endoscopic (Kumar et al, 2004).
Endoscopic Biliary Decompression (See Chapters 27 and 36)
In cases of difficult biliary cannulation, an endoscopic sphincterotomy (EST) may be performed. A common EST technique is to perform a high-frequency electric surgical incision of the duodenal papilla, using a sphincterotome selectively inserted into the bile duct. In contrast to EST for stone removal, when performed for drainage, only a limited incision is necessary. Bleeding, retroduodenal perforation, and pancreatitis are the most common complications after ERCP and sphincterotomy. Morbidity and mortality rates are much higher with sphincterotomy than with nasobiliary catheter drainage when patients are critically ill (Boender et al, 1995; Chawla et al, 1994; Leese et al, 1986). Complications commonly reported with EST are hemorrhage (2% to 3%) and pancreatitis (1.9% to 5.4%), and mortality rate is 0.4% to 1.3% (Cotton et al, 1991; Freeman et al, 1996).
Contraindications
As stated, emergent sphincterotomy to decompress the bile duct should be avoided in septic patients, and especially in elderly patients, because of potentially high mortality rates (18.8%). In the elderly, endoscopic drainage alone has been observed to result in lower morbidity (16.7%) and mortality (5.6%) rates than surgical (87.5% and 25.0%, respectively) or percutaneous drainage (36.4% and 9.1%, respectively) (Boender et al, 1995; Sugiyama & Atomi, 1997). It is important to emphasize that once control of sepsis has been achieved, sphincterotomy and more optimal biliary imaging can be obtained, thus defining the underlying obstruction and enabling definitive treatment for either a stone, tumor, or stricture (Lee et al, 2002).