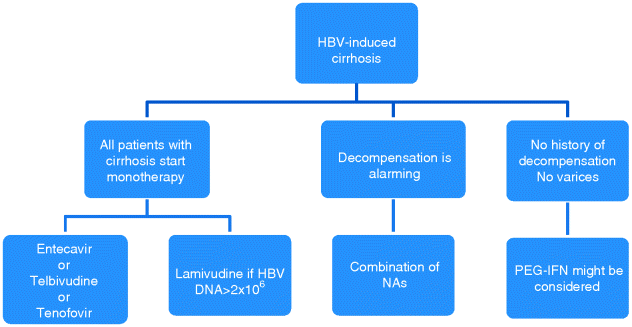
Chapter 24 Gamal Esmat and Maissa El Raziky Faculty of Medicine, Cairo University, Cairo, Egypt When the term cirrhosis was coined two centuries ago by Laennec, it meant – by definition – an end-stage irreversible liver disease. Nowadays this word encompasses a whole range of disorders including some degree of reversibility [1]. The issue of regression or reversal of cirrhosis was first noticed in animal models upon the discontinuation of injurious agents to the liver or initiating treatment with antifibrotic agents [2], but this regression was not fully demonstrated in humans. Evidence of fibrotic and/or cirrhotic regression were reported in chronic viral hepatitis [3–5], alcoholic and nonalcoholic steatohepatitis [6,7], and autoimmune hepatitis [8]. However, these studies concluded that, in spite of a variable degree of fibrosis regression there was no complete reversal of cirrhosis [3–8]. In hepatitis B virus (HBV) related cirrhosis, the use of nucleot(s)ide analogs showed advantages for Child–Turcotte–Pugh (CTP) score improvement and transplant-free survival, and the incidence of hepatocellular carcinoma (HCC) was reduced [9]. Interferon treatment for patients with hepatitis C virus (HCV) related cirrhosis inhibits the development of HCC [10] and improves survival [11]. In situations where treating the underlying process is not possible, specific antifibrotic therapy is highly recommended [12]. Treatment is mandatory in patients with compensated or decompensated cirrhosis and detectable HBV DNA, independently of alanine aminotransferase (ALT) levels. Patients with no HBV DNA detectable by current sensitive tests should be monitored. Before treatment, the following factors should be considered: age, HBV DNA levels, hepatic performance, whether the cirrhosis is compensated or decompensated, presence of esophagogastric varices, comorbidities and cofactors potentially worsening liver disease, and the prospect of liver transplantation (LT) [13]. Nucleot(s)ide analog therapy can achieve sustained suppression of HBV replication, remission of liver disease, and has been shown to halt disease progression and prevent long-term complications, such as decompensation and HCC [9]. Since its introduction in the 1990s, lamivudine has been considered as a safe and effective drug in suppression of HBV replication [14]. It was reported that in patients with compensated cirrhosis, prolonged therapy with lamivudine was well tolerated and resulted in improved serum biochemical values and loss of HBV DNA together with clinical improvements in the form of decrease in CTP scores, an improvement in albumin, and a reduced incidence of ascites [15]. However, reports of long-term antiviral treatment resistance and selection of antiviral-resistant mutations with subsequent reduction of clinical benefit may be associated with flares of the disease; even frank hepatic decompensation was frequently described with lamivudine [16]. Thus, other treatment strategies, such as add-on therapies with another agent that lacks cross-resistance, are most often necessary for suppression of viral replication and prevention of further worsening of disease [17]. Adefovir was the first nucleotide analog to be used in cases of lamivudine resistance [18]. A number of studies have clearly demonstrated that adding adefovir to ongoing lamivudine was associated with a lower risk of adefovir-resistance than switching to adefovir in both hepatitis Be antigen (HBeAg)-positive and HbeAg-negative patients with lamivudine-resistant HBV [19,20]. Current guidelines recommend that when adefovir is used as an add-on therapy in patients with lamivudine-resistant HBV infection, lamivudine should be continued indefinitely to decrease the risk of hepatitis flares during the transition period and to reduce the risk of subsequent adefovir resistance [21]. Randomized, placebo-controlled studies demonstrated that this add-on therapy was safe and associated with substantial virologic and biochemical improvement after 1–2 months of treatment [17]. The European Association for the Study of the Liver (EASL) guidelines recommend that in lamivudine-resistant YMDD mutations, tenofovir monotherapy should be started [22]. Among entecavir-treated patients with advanced liver fibrosis, improvement in fibrosis was observed in almost 60% of nucleos(t)ide-naive HBeAg-positive or HBeAg-negative patients. The treatment was well tolerated. The performance of entecavir relative to that of lamivudine in patients with advanced liver fibrosis and/or cirrhosis was comparable with the relationship observed in noncirrhotic patients. Virologic response to entecavir is associated with a lower probability of disease progression in patients with cirrhosis, even after correction for possible baseline confounders. When using a threshold of 2000 IU/mL, the association between viral replication and disease progression was reduced, suggesting that complete viral suppression is essential for nucleos(t)ide analog treatment, especially in patients with cirrhosis, whether compensated or decompensated [23,24]. For chronic hepatitis B (CHB) patients with cirrhosis, telbivudine can improve CTP score at 48 weeks of treatment. One of the major drawbacks with long-term telbivudine treatment is the development of drug resistance [25]. Tenofovir is considered one of the first line drugs for treatment-naïve patients. Having a potent antiviral activity, low resistance rate and toxicity, it can be used in the long term for effective HBV DNA suppression. Tenofovir is useful in patients with lamivudine, telbivudine, and entecavir resistance; it is cost-effective for patients with cirrhotic CHB [26]. CHB patients with advanced fibrosis are often not considered for treatment with pegylated interferon (PEG-IFN) because IFN therapy may precipitate immunologic flares, potentially inducing hepatic decompensation. Treating HBeAg-positive CHB patients with advanced fibrosis and/or cirrhosis with compensated liver disease, with 52 weeks of PEG-IFN-α-2b (100 μg/week) alone or in combination with lamivudine (100 mg/day), resulted in virologic response in about 25% of patients. Improvement in liver fibrosis occurred in two-thirds of patients. The authors concluded that PEG-IFN is effective and safe for HBeAg-positive patients with advanced fibrosis. Patients with advanced fibrosis or cirrhosis but compensated liver disease should not be excluded from PEG-IFN treatment [27]. Patients with decompensated HBV cirrhosis at initial presentation have a poor short-term prognosis, with an estimated 5-year survival of only 10–15% [4]. Although LT is an effective treatment option for decompensated HBV cirrhosis, shortage of donor organs and limited availability of resources worldwide precludes the majority of HBV patients in endemic areas from undergoing transplantation, raising the issue of considering antiviral therapy as an alternative option [28]. Pooled 1-year data of treating patients with decompensated cirrhosis showed benefit favoring lamivudine vs. untreated controls for CTP score improvement by at least 2 points and transplant-free survival [14]. Adefovir monotherapy was less successful in achieving undetectable HBV DNA at 1-year in 41% than with lamivudine and entecavir. Overall, 1-year transplant-free survival rates varied from 78% with lamivudine to 95% and 94% with tenofovir and telbivudine, respectively [9]. The 1-year incidence of drug-resistant HBV was almost null with adefovir, entecavir, and tenofovir and around 10% with lamivudine although telbivudine was associated with almost 30% incidence at 2 years. Drug-related adverse events were infrequently reported. All the oral antiviral agents were associated with improved virologic, biochemical, and clinical parameters at 1 year. However, the efficacy of lamivudine is limited by drug resistance, and adefovir is limited by its slower onset of action [29]. Other beneficial effects with the oral nucleo(t)side agents included removal from the LT waiting list in 6% of patients receiving adefovir, 21% receiving lamivudine, and 11% treated with entecavir. Development of HCC at 1 year was reported to be lower in treated patients. However, lamivudine was not more effective in reducing the incidence of HCC at 1 year compared to untreated patients [9]. The largest amount of data involved lamivudine monotherapy in decompensated HBV cirrhosis, presumably due to the fact that this agent has been available for over 15 years in many countries. Resistance to lamivudine, which occurred in 10% of patients at 1 year, has emerged as a major limitation especially in patients with advanced disease who cannot tolerate biochemical flares with emergence of drug-resistant HBV. HBV cirrhosis patients had a higher rate of clinical outcomes when they developed drug resistance during follow-up and a higher mortality rate [14]. The data of entecavir monotherapy in patients with decompensated HBV cirrhosis showed no evidence of entecavir-resistant HBV at 1 year despite the presence of lamivudine-resistant HBV in some of these patients [24,30,31]. Data on the use of tenofovir and telbivudine in decompensated HBV cirrhosis are restricted by the small numbers of studies for each drug. Tenofovir was as effective and potent as entecavir with a similar side-effect profile and no evidence of drug-resistance. Tenofovir-treated patients were able to achieve HBeAg seroconversion [32,33]. Drug safety is an important consideration when treating patients with decompensated cirrhosis who have impaired drug metabolism, protein binding, and renal functions. Lamivudine had the fewest number of serious adverse effects. Renal insufficiency with associated renal tubular dysfunction was reported in adefovir, tenofovir, or entecavir-treated decompensated patients, especially in those with low baseline glomerular filtration rate (GFR) [9]. Recent studies have demonstrated an increasing frequency of serum creatine phosphokinase (CPK) elevations with prolonged tenofovir use. Thus, telbivudine may be a second line agent for patients with decompensated HBV cirrhosis due to safety concerns. There are also some concerns regarding the long-term safety of tenofovir in patients with malnutrition and low vitamin D levels. Another concern with the prolonged use of nucleos(t)ide analogs is the potential for mitochondrial toxicity and lactic acidosis, which may present with myopathy, neuropathy, and rhabdomyolysis [34]. HBV-related acute-on-chronic liver failure (ACLF) has an extremely poor prognosis. The 3-month survival rate was higher with those treated with entecavir or lamivudine than in nontreated patients. Short-term antiviral treatment with entecavir or lamivudine rapidly suppressed HBV replication, increased the short-term survival rate, and reduced recurrence. Early administration of long-term nucleoside analog treatment is recommended for the prevention of recurrence of HBV-related ACLF [35]. In patients eligible for LT, treatment with nucleoside analogs should be started in collaboration with a reference transplant center. This should aim to control the risk of clinical deterioration, reduce viremia to as low as possible before transplantation to reduce the risk of HBV reactivation, and prevent the emergence of HBV-resistant mutants (Figure 24.1). After transplantation, the standard of prophylaxis is a combination of nucleoside analogs and anti-HBV immunoglobulins (HBIG). Post-LT experience has been mostly reported with lamivudine and/or adefovir. Data on entecavir, tenofovir, and telbivudine are rare [36]. Figure 24.1 Algorithm for the treatment of hepatitis B virus (HBV) induced cirrhosis. NA, nucleot(s)ide analog. The clinical significance of treating patients with established cirrhosis has been a matter of debate. Earlier studies on the treatment of HCV-related cirrhosis failed to demonstrate the benefit of viral eradication in subjects with advanced liver disease [37,38]. However, retrospective, longitudinal study of cirrhotic patients treated with IFN, and who were followed-up for 5 years, showed that the annual incidence of HCC was 2.3% for untreated and 1.0% for treated patients, whereas the incidence of hepatic decompensation was 1.5% and 5.7%, respectively, which supports the claim that eradication of HCV infection prevents progression to potentially fatal complications by fibrosis regression and lowering portal hypertension, thus effectively confronting the debate of reversibility of cirrhosis [39,40]. The analysis of four randomized trials, pooling data of 3010 treatment-naive patients treated with different regimens of standard IFN-α or PEG-IFN-α, with or without ribavirin, demonstrated the reversal of cirrhosis in half of patients with baseline cirrhosis. Cirrhotic patients who have cleared HCV infection showed lower rates of liver decompensation, HCC development, and liver-related death [11]. Another international, multicenter, long-term follow-up study from five large hospitals in Canada and Europe of 530 patients with chronic HCV infection who received an IFN-based treatment concluded that sustained virologic response (SVR) was associated with lower all-cause mortality [41]. Anti-HCV treatment in cirrhotic patients is less effective than in noncirrhotics. Based on effectiveness and tolerability reports, therapy has a significant effect in patients with compensated cirrhosis, while decompensated patients need to re-evaluate the risks versus benefits of treatment [42].
Changing Outcomes with Antiviral or Antifibrotic Therapies
Introduction
HBV-Related Cirrhosis
Nucleos(t)ide Analogs in Compensated Cirrhosis
Nucleos(t)ide Analogs in Decompensated Cirrhosis
Hepatitis B Virus-Related Acute-on-Chronic Liver Failure
Hepatitis B Virus-Related Liver Transplantation
HCV-Related Cirrhosis
Anti-HCV Treatment in Compensated Cirrhosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







